Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice
- PMID: 16049327
- PMCID: PMC1603552
- DOI: 10.1016/s0002-9440(10)62985-9
Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice
Abstract
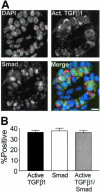
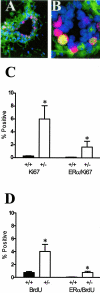
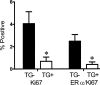
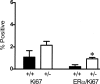
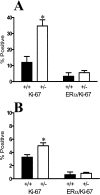
Transforming growth factor (TGF)-beta1 is a potent inhibitor of mammary epithelial proliferation. In human breast, estrogen receptor (ER)-alpha cells rarely co-localize with markers of proliferation, but their increased frequency correlates with breast cancer risk. To determine whether TGF-beta1 is necessary for the quiescence of ER-alpha-positive populations, we examined mouse mammary epithelial glands at estrus. Approximately 35% of epithelial cells showed TGF-beta1 activation, which co-localized with nuclear receptor-phosphorylated Smad 2/3, indicating that TGF-beta signaling is autocrine. Nuclear Smad co-localized with nuclear ER-alpha. To test whether TGF-beta inhibits proliferation, we examined genetically engineered mice with different levels of TGF-beta1. ER-alpha co-localization with markers of proliferation (ie, Ki-67 or bromodeoxyuridine) at estrus was significantly increased in the mammary glands of Tgf beta1 C57/bl/129SV heterozygote mice. This relationship was maintained after pregnancy but was absent at puberty. Conversely, mammary epithelial expression of constitutively active TGF-beta1 via the MMTV promoter suppressed proliferation of ER-alpha-positive cells. Thus, TGF-beta1 activation functionally restrains ER-alpha-positive cells from proliferating in adult mammary gland. Accordingly, we propose that TGF-beta1 dysregulation may promote proliferation of ER-alpha-positive cells associated with breast cancer risk in humans.
Figures

Similar articles
-
Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation.Am J Pathol. 2002 Jun;160(6):2081-93. doi: 10.1016/s0002-9440(10)61158-3. Am J Pathol. 2002. PMID: 12057913 Free PMC article.
-
Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study.Cancer Res. 2002 Jan 15;62(2):497-505. Cancer Res. 2002. PMID: 11809701
-
Dominant-negative interference of the transforming growth factor beta type II receptor in mammary gland epithelium results in alveolar hyperplasia and differentiation in virgin mice.Cell Growth Differ. 1998 Mar;9(3):229-38. Cell Growth Differ. 1998. PMID: 9543389
-
TGF beta regulation of cell proliferation.Princess Takamatsu Symp. 1994;24:250-63. Princess Takamatsu Symp. 1994. PMID: 8983080 Review.
-
Hormone-sensing mammary epithelial progenitors: emerging identity and hormonal regulation.J Mammary Gland Biol Neoplasia. 2015 Jun;20(1-2):75-91. doi: 10.1007/s10911-015-9344-1. Epub 2015 Sep 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26390871 Review.
Cited by
-
miR-30a-3p Regulates Autophagy in the Involution of Mice Mammary Glands.Int J Mol Sci. 2023 Sep 20;24(18):14352. doi: 10.3390/ijms241814352. Int J Mol Sci. 2023. PMID: 37762652 Free PMC article.
-
A multiscale statistical mechanical framework integrates biophysical and genomic data to assemble cancer networks.Nat Genet. 2014 Dec;46(12):1363-1371. doi: 10.1038/ng.3138. Epub 2014 Nov 2. Nat Genet. 2014. PMID: 25362484 Free PMC article.
-
Transcriptome analysis of the hormone-sensing cells in mammary epithelial reveals dynamic changes in early pregnancy.BMC Dev Biol. 2015 Jan 27;15:7. doi: 10.1186/s12861-015-0058-9. BMC Dev Biol. 2015. PMID: 25623114 Free PMC article.
-
Heterozygosity for TGF β1 -509C/T Polymorphism is associated with risk for breast cancer in South Indian population.Tumour Biol. 2013 Feb;34(1):99-105. doi: 10.1007/s13277-012-0516-y. Epub 2012 Sep 22. Tumour Biol. 2013. PMID: 23001908
-
ER and PR signaling nodes during mammary gland development.Breast Cancer Res. 2012 Jul 19;14(4):210. doi: 10.1186/bcr3166. Breast Cancer Res. 2012. PMID: 22809143 Free PMC article. Review.
References
-
- Daniel CW, Robinson SD. Regulation of mammary growth and function by TGF-β. Mol Reprod Dev. 1992;32:145–151. - PubMed
-
- Derynck R, Ackhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. - PubMed
-
- Wakefield L, Colletta AA, McCune BK, Sporn MB. Roles for transforming growth factors-β in the genesis, prevention and treatment of breast cancer. Dickson RB, Lippman ME, editors. Boston: Kluwer Academic Publishers,; Genes, Oncogens, and HormonesAdvances in Cellular and Molecular Biology of Breast Cancer. 1991:pp 97–136. - PubMed
-
- Smith GH. TGF-β and functional differentiation. J Mammary Gland Biol Neoplasia. 1996;1:343–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

