Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance
- PMID: 16049329
- PMCID: PMC1603568
- DOI: 10.1016/s0002-9440(10)62987-2
Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance
Abstract
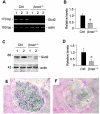
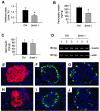
Hepatocyte growth factor (HGF) and its c-met receptor consist of a paired signaling system that has been implicated in the regulation of pancreatic beta-cell survival, proliferation, and function. To define the role of HGF/c-met signaling in beta-cell biology in vivo, we have generated conditional knockout mice in which the c-met receptor gene was specifically inactivated in pancreatic beta cells by the Cre-loxP system. Mice with beta-cell-specific deletion of the c-met receptor (betamet-/-) displayed slight growth retardation, mild hyperglycemia, and decreased serum insulin levels at 6 months of age when compared with their control littermates. Deficiency of the c-met receptor in beta cells resulted in a complete loss of acute-phase insulin secretion in response to glucose and an impaired glucose tolerance. Glucose transporter-2 expression was down-regulated in the beta cells of betamet-/- mice. Compared to controls, betamet-/- mice exhibited reduced islet size and decreased insulin content in the pancreas, but displayed normal islet morphology. Therefore, HGF/c-met signaling plays an imperative role in controlling islet growth, in regulating beta-cell function, and in maintaining glucose homeostasis.
Figures



Similar articles
-
Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass.Diabetes. 2005 Jul;54(7):2090-102. doi: 10.2337/diabetes.54.7.2090. Diabetes. 2005. PMID: 15983210
-
Overexpression of dominant-negative mutant hepatocyte nuclear fctor-1 alpha in pancreatic beta-cells causes abnormal islet architecture with decreased expression of E-cadherin, reduced beta-cell proliferation, and diabetes.Diabetes. 2002 Jan;51(1):114-23. doi: 10.2337/diabetes.51.1.114. Diabetes. 2002. PMID: 11756330
-
Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes.Diabetes. 2011 Feb;60(2):525-36. doi: 10.2337/db09-1305. Epub 2010 Oct 27. Diabetes. 2011. PMID: 20980460 Free PMC article.
-
Mechanisms of glucose sensing and multiplicity of glucose sensors.Ann Endocrinol (Paris). 2004 Feb;65(1):9-12. doi: 10.1016/s0003-4266(04)95624-7. Ann Endocrinol (Paris). 2004. PMID: 15122086 Review. No abstract available.
-
Glucose Homeostasis and Pancreatic Islet Size Are Regulated by the Transcription Factors Elk-1 and Egr-1 and the Protein Phosphatase Calcineurin.Int J Mol Sci. 2023 Jan 3;24(1):815. doi: 10.3390/ijms24010815. Int J Mol Sci. 2023. PMID: 36614256 Free PMC article. Review.
Cited by
-
Partial-Hepatectomized (70%) Model Shows a Correlation between Hepatocyte Growth Factor Levels and Beta-Cell Mass.Front Endocrinol (Lausanne). 2015 Feb 16;6:20. doi: 10.3389/fendo.2015.00020. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25762981 Free PMC article. No abstract available.
-
The MET Receptor Tyrosine Kinase Confers Repair of Murine Pancreatic Acinar Cells following Acute and Chronic Injury.PLoS One. 2016 Oct 31;11(10):e0165485. doi: 10.1371/journal.pone.0165485. eCollection 2016. PLoS One. 2016. PMID: 27798657 Free PMC article.
-
Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury.Kidney Int. 2013 Sep;84(3):509-20. doi: 10.1038/ki.2013.102. Epub 2013 May 29. Kidney Int. 2013. PMID: 23715119 Free PMC article.
-
Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation.Am J Pathol. 2006 May;168(5):1500-12. doi: 10.2353/ajpath.2006.050747. Am J Pathol. 2006. PMID: 16651617 Free PMC article.
-
Loss of sugar detection by GLUT2 affects glucose homeostasis in mice.PLoS One. 2007 Dec 12;2(12):e1288. doi: 10.1371/journal.pone.0001288. PLoS One. 2007. PMID: 18074013 Free PMC article.
References
-
- Marx J. Unraveling the causes of diabetes. Science. 2002;296:686–689. - PubMed
-
- Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–C14. - PubMed
-
- Garcia-Ocana A, Vasavada RC, Takane KK, Cebrian A, Lopez-Talavera JC, Stewart AF. Using beta-cell growth factors to enhance human pancreatic Islet transplantation. J Clin Endocrinol Metab. 2001;86:984–988. - PubMed
-
- Dai C, Li Y, Yang J, Liu Y. Hepatocyte growth factor preserves beta cell mass and mitigates hyperglycemia in streptozotocin-induced diabetic mice. J Biol Chem. 2003;278:27080–27087. - PubMed
-
- Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera JC, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem. 2003;278:343–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

