Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts
- PMID: 16049333
- PMCID: PMC1603576
- DOI: 10.1016/S0002-9440(10)62991-4
Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts
Abstract
Stromagenesis is a host reaction of connective tissue that, when induced in cancer, produces a progressive and permissive mesenchymal microenvironment, thereby supporting tumor progression. The stromal microenvironment is complex and comprises several cell types, including fibroblasts, the primary producers of the noncellular scaffolds known as extracellular matrices. The events that support tumor progression during stromagenesis are for the most part unknown due to the lack of suitable, physiologically relevant, experimental model systems. In this report, we introduce a novel in vivo-like three-dimensional system derived from tumor-associated fibroblasts at diverse stages of tumor development that mimic the stromagenic features of fibroblasts and their matrices observed in vivo. Harvested primary stromal fibroblasts, obtained from different stages of tumor development, did not retain in vivo stromagenic characteristics when cultured on traditional two-dimensional substrates. However, they were capable of effectively maintaining the tumor-associated stromal characteristics within three-dimensional cultures. In this study, we demonstrate that in vivo-like three-dimensional matrices appear to have the necessary topographical and molecular information sufficient to induce desmoplastic stroma differentiation of normal fibroblasts.
Figures




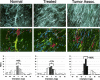
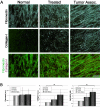
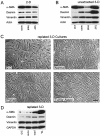
References
-
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): active stromal participants in tumor development and progression? Histol Histopathol. 2002;17:599–621. - PubMed
-
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part II): functional impact on tumor tissue. Histol Histopathol. 2002;17:623–637. - PubMed
-
- Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA. The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int J Cancer. 2004;108:173–180. - PubMed
-
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

