Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein
- PMID: 16049335
- PMCID: PMC1603574
- DOI: 10.1016/s0002-9440(10)62993-8
Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein
Abstract
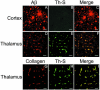
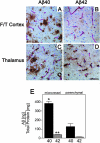
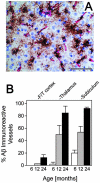
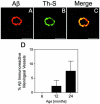
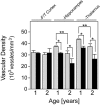
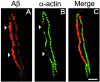
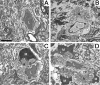
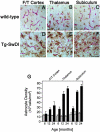
Cerebral vascular amyloid beta-protein (Abeta) deposition, also known as cerebral amyloid angiopathy, is a common pathological feature of Alzheimer's disease. Additionally, several familial forms of cerebral amyloid angiopathy exist including the Dutch (E22Q) and Iowa (D23N) mutations of Abeta. Increasing evidence has associated cerebral microvascular amyloid deposition with neuroinflammation and dementia in these disorders. We recently established a transgenic mouse model (Tg-SwDI) that expresses human vasculotropic Dutch/Iowa mutant amyloid beta-protein precursor in brain. Tg-SwDI mice were shown to develop early-onset deposition of Abeta exhibiting high association with cerebral microvessels. Here we present quantitative temporal analysis showing robust and progressive accumulation of cerebral microvascular fibrillar Abeta accompanied by decreased cerebral vascular densities, the presence of apoptotic cerebral vascular cells, and cerebral vascular cell loss in Tg-SwDI mice. Abundant neuroinflammatory reactive astrocytes and activated microglia strongly associated with the cerebral microvascular fibrillar Abeta deposits. In addition, Tg-SwDI mouse brain exhibited elevated levels of the inflammatory cytokines interleukin-1beta and -6. Together, these studies identify the Tg-SwDI mouse as a unique model to investigate selective accumulation of cerebral microvascular amyloid and the associated neuroinflammation.
Figures


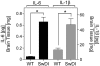
References
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Jellinger KA. Alzheimer’s disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. - PubMed
-
- Vinters HV, Farag ES. Amyloidosis of cerebral arteries. Adv Neurol. 2003;92:105–112. - PubMed
-
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GTAM, Luyendijk W, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

