A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis
- PMID: 16050959
- PMCID: PMC1185540
- DOI: 10.1186/1471-2334-5-62
A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis
Abstract
Background: Mycobacterium tuberculosis is a leading cause of death worldwide. In multi-drug resistant tuberculosis (MDR-TB) infectiousness is frequently prolonged, jeopardizing efforts to control TB. The conventional tuberculosis drug susceptibility tests are sensitive and specific, but they are not rapid. The INNO-LiPA Rif. TB (LiPA) is a commercial line probe assay designed to rapidly detect rifampicin resistance, a marker of MDR-TB. Although LiPA has shown promising results, its overall accuracy has not been systematically evaluated.
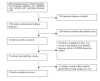
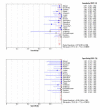
Methods: We did a systematic review and meta-analysis to evaluate the accuracy of LiPA for the detection of rifampicin-resistant tuberculosis among culture isolates and clinical specimens. We searched Medline, Embase, Web of Science, BIOSIS, and Google Scholar, and contacted authors, experts and the manufacturer. Fifteen studies met our inclusion criteria. Of these, 11 studies used culture isolates, one used clinical specimens, and three used both. We used a summary receiver operating characteristic (SROC) curve and Q* index to perform meta-analysis and summarize diagnostic accuracy.
Results: Twelve of 14 studies that applied LiPA to isolates had sensitivity greater than 95%, and 12 of 14 had specificity of 100%. The four studies that applied LiPA directly to clinical specimens had 100% specificity, and sensitivity that ranged between 80% and 100%. The SROC curve had an area of 0.99 and Q* of 0.97.
Conclusion: LiPA is a highly sensitive and specific test for the detection of rifampicin resistance in culture isolates. The test appears to have relatively lower sensitivity when used directly on clinical specimens. More evidence is needed before LiPA can be used to detect MDR-TB among populations at risk in clinical practice.
Figures

Similar articles
-
Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance.Cochrane Database Syst Rev. 2018 Aug 27;8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Jan 15;1:CD012768. doi: 10.1002/14651858.CD012768.pub3. PMID: 30148542 Free PMC article. Updated.
-
The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis.J Antimicrob Chemother. 2008 Jul;62(1):56-64. doi: 10.1093/jac/dkn139. Epub 2008 Apr 10. J Antimicrob Chemother. 2008. PMID: 18407918
-
Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis.J Antimicrob Chemother. 2007 Feb;59(2):175-83. doi: 10.1093/jac/dkl477. Epub 2006 Nov 28. J Antimicrob Chemother. 2007. PMID: 17135182
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2022 Sep 6;9(9):CD013359. doi: 10.1002/14651858.CD013359.pub3. Cochrane Database Syst Rev. 2022. PMID: 36065889 Free PMC article.
-
Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin.Cochrane Database Syst Rev. 2022 May 18;5(5):CD014841. doi: 10.1002/14651858.CD014841.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583175 Free PMC article.
Cited by
-
Diagnosis of multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: Current standards and challenges.Can J Infect Dis Med Microbiol. 2008 Mar;19(2):169-72. doi: 10.1155/2008/857901. Can J Infect Dis Med Microbiol. 2008. PMID: 19352448 Free PMC article.
-
Mycobacterium tuberculosis thymidylate kinase antigen assays for designating incipient, high-risk latent M.tb infection.BMC Infect Dis. 2018 Mar 16;18(1):133. doi: 10.1186/s12879-018-3007-y. BMC Infect Dis. 2018. PMID: 29548281 Free PMC article.
-
Molecular diagnostic techniques.Medicine (Abingdon). 2009 Oct;37(10):535-540. doi: 10.1016/j.mpmed.2009.07.012. Epub 2009 Sep 19. Medicine (Abingdon). 2009. PMID: 32288568 Free PMC article. Review.
-
Role of the clinical mycobacteriology laboratory in diagnosis and management of tuberculosis in low-prevalence settings.J Clin Microbiol. 2011 Mar;49(3):772-6. doi: 10.1128/JCM.02451-10. Epub 2010 Dec 22. J Clin Microbiol. 2011. PMID: 21177899 Free PMC article. Review.
-
Clinical Impact on Tuberculosis Treatment Outcomes of Discordance Between Molecular and Growth-Based Assays for Rifampin Resistance, California 2003-2013.Open Forum Infect Dis. 2016 Aug 24;3(3):ofw150. doi: 10.1093/ofid/ofw150. eCollection 2016 Sep. Open Forum Infect Dis. 2016. PMID: 27704008 Free PMC article.
References
-
- The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance . Anti-Tuberculosis drug resistance in the world, report number 2. Geneva; 2004.
-
- Heifets LB, Cangelosi GA. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int J Tuberc Lung Dis. 1999;3:564–581. - PubMed
-
- Cavusoglu C, Hilmioglu S, Guneri S, Bilgic A. Characterization of rpo B mutations in Rifampin-resistant clinical isolates of Mycobacterium tuberculosis from Turkey by DNA sequencing and line probe assay. J Clin Microbiol. 2002;40:4435–4438. doi: 10.1128/JCM.40.12.4435-4438.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

