Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer
- PMID: 16050980
- PMCID: PMC4466120
- DOI: 10.1016/j.mito.2005.02.001
Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer
Abstract
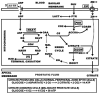
Human prostate secretory epithelial cells have the uniquely specialized function of accumulating and secreting extremely high levels of citrate. This is achieved by their ability to accumulate high cellular levels of zinc that inhibit citrate oxidation. This process of net citrate production requires unique metabolic/bioenergetic mitochondrial relationships. In prostate cancer, the malignant cells undergo a metabolic transformation from zinc-accumulating citrate-producing sane cells to citrate-oxidizing malignant cells that lost the ability to accumulate zinc. This review describes the metabolic/bioenergetic, zinc and mitochondrial relationships involved in normal and malignant prostate. Hopefully, this report will generate much needed interest and research in this neglected, but critically important, area of investigation.
Figures
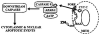


References
-
- Argiles JM, Lopez-Soriano FJ. Why do cancer cells have such a high glycolytic rate. Med. Hypotheses. 1990;32:151–155. - PubMed
-
- Baggetto LG. Deviant energetic metabolism of glycolytic cancer cells. Biochimie. 1992;74:959–974. - PubMed
-
- Barron ESG, Huggins C. The metabolism of isolated prostatic tissue. J. Urol. 1944;51:630–634.
-
- Brierley GP, Knight VA. Ion transport by heart mitochondria. X. The uptake and release of Zn2+ and its relation to the energy-linked accumulation of magnesium. Biochemistry. 1967;6:3892–3901. - PubMed
-
- Costello LC, Franklin RB. Aconitase activity, citrate oxidation and zinc inhibition in rat ventral prostate. Enzyme. 1981;26:281–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

