Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs
- PMID: 16051625
- PMCID: PMC1464254
- DOI: 10.1113/jphysiol.2005.093153
Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs
Abstract
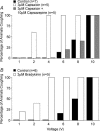
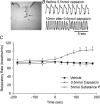
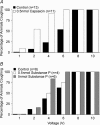
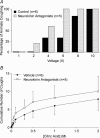
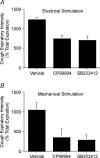
Cough initiated from the trachea and larynx in anaesthetized guinea-pigs is mediated by capsaicin-insensitive, mechanically sensitive vagal afferent neurones. Tachykinin-containing, capsaicin-sensitive C-fibres also innervate the airways and have been implicated in the cough reflex. Capsaicin-sensitive nerves act centrally and synergistically to modify reflex bronchospasm initiated by airway mechanoreceptor stimulation. The hypothesis that polymodal mechanoreceptors and capsaicin-sensitive afferent nerves similarly interact centrally to regulate coughing was addressed in this study. Cough was evoked from the tracheal mucosa either electrically (16 Hz, 10 s trains, 1-10 V) or by citric acid (0.001-2 m). Neither capsaicin nor bradykinin evoked a cough when applied to the trachea of anaesthetized guinea-pigs, but they substantially reduced the electrical threshold for initiating the cough reflex. The TRPV1 receptor antagonist capsazepine prevented the increased cough sensitivity induced by capsaicin. These effects of topically applied capsaicin and bradykinin were not due to interactions between afferent nerve subtypes within the tracheal wall or a direct effect on the cough receptors, as they were mimicked by nebulizing 1 mg ml(-1) bradykinin into the lower airways and by microinjecting 0.5 nmol capsaicin into nucleus of the solitary tract (nTS). Citric acid-induced coughing was also potentiated by inhalation of bradykinin. The effects of tracheal capsaicin challenge on cough were mimicked by microinjecting substance P (0.5-5 nmol) into the nTS and prevented by intracerebroventricular administration (20 nmol h(-1)) of the neurokinin receptor antagonists CP99994 or SB223412. Tracheal application of these antagonists was without effect. C-fibre activation may thus sensitize the cough reflex via central mechanisms.
Figures
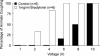

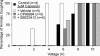
References
-
- Barbieri M, Nistri A. Depression of windup of spinal neurons in the neonatal rat spinal cord in vitro by an NK3 tachykinin receptor antagonist. J Neurophysiol. 2001;85:1502–1511. - PubMed
-
- Baude A, Shigemoto R. Cellular and subcellular distribution of substance P receptor immunoreactivity in the dorsal vagal complex of the rat and cat: a light and electron microscope study. J Comp Neurol. 1998;402:181–196. - PubMed
-
- Bergren DR. Sensory receptor activation by mediators of defense reflexes in guinea-pig lungs. Respir Physiol. 1997;108:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

