A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders
- PMID: 16051700
- PMCID: PMC1183597
- DOI: 10.1073/pnas.0505149102
A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders
Abstract
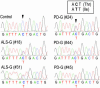
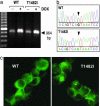
Guamanian amyotrophic lateral sclerosis (ALS-G) and parkinsonism dementia (PD-G) have been epidemiologically linked to an environment severely deficient in calcium (Ca2+) and magnesium (Mg2+). Transient receptor potential melastatin 7 (TRPM7) is a bifunctional protein containing both channel and kinase domains that has been proposed to be involved in the homeostatic regulation of intracellular Ca2+, Mg2+, and trace metal ion concentration. There is evidence that TRPM7 is constitutively active and that the number of available channels is dependent on intracellular free Mg2+ levels. We found a TRPM7 variant in a subset of ALS-G and PD-G patients that produces a protein with a missense mutation, T1482I. Recombinant T1482I TRPM7 exhibits the same kinase catalytic activity as WT TRPM7. However, heterologously expressed T1482I TRPM7 produces functional channels that show an increased sensitivity to inhibition by intracellular Mg2+. Because the incidence of ALS-G and PD-G has been associated with prolonged exposure to an environment severely deficient in Ca2+ and Mg2+, we propose that this variant TRPM7 allele confers a susceptibility genotype in such an environment. This study represents an initial attempt to address the important issue of gene-environment interactions in the etiology of these diseases.
Figures



References
-
- Garruto, R. M. (1991) Neurotoxicology 9, 347-378. - PubMed
-
- Bailey-Wilson, J. E., Plato, C. C., Elston, R. C. & Garruto, R. M. (1992) Am. J. Med. Genet. 45, 68-76. - PubMed
-
- Plato, C. C., Garruto, R. M., Galasko, D., Craig, U. K, Plato, M., Gamst, A., Torres, J. M. & Wiederholt, W. (2003) Am. J. Epidemiol. 157, 149-157. - PubMed
-
- Plato, C. C., Galasko, D., Garruto, R. M., Plato, M., Gamst, A., Torres, J. M. & Wiederholt, W. (2002) Neurology 58, 765-773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

