Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain
- PMID: 16051701
- PMCID: PMC1181239
- DOI: 10.1073/pnas.0504926102
Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain
Abstract
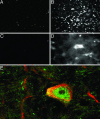
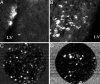
This article reports on the application of organically modified silica (ORMOSIL) nanoparticles as a nonviral vector for efficient in vivo gene delivery. Highly monodispersed, stable aqueous suspension of nanoparticles, surface-functionalized with amino groups for binding of DNA, were prepared and characterized. Stereotaxic injections of nanoparticles, complexed with plasmid DNA encoding for EGFP, into the mouse ventral midbrain and into lateral ventricle, allowed us to fluorescently visualize the extensive transfection of neuronal-like cells in substantia nigra and areas surrounding the lateral ventricle. No ORMOSIL-based toxicity was observed 4 weeks after transfection. The efficiency of transfection equaled or exceeded that obtained in studies using a viral vector. An in vivo optical imaging technique (a fiber-based confocal fluorescent imaging system) provided an effective means to show the retention of viability of the transfected cells. The ORMOSIL-mediated transfections also were used to manipulate the biology of the neural stem/progenitor cells in vivo. Transfection of a plasmid expressing the nucleus-targeting fibroblast growth factor receptor type 1 resulted in significant inhibition of the in vivo incorporation of bromodeoxyuridine into the DNA of the cells in the subventricular zone and the adjacent rostral migratory stream. This in vivo approach shows that the nuclear receptor can control the proliferation of the stem/progenitor cells in this region of the brain. The results of this nanomedicine approach using ORMOSIL nanoparticles as a nonviral gene delivery platform have a promising future direction for effective therapeutic manipulation of the neural stem/progenitor cells as well as in vivo targeted brain therapy.
Figures



References
-
- Prasad, P. N. (2003) Introduction to Biophotonics (Wiley, New York).
-
- Prasad, P. N. (2004) Nanophotonics (Wiley, New York).
-
- Luo, D. & Saltzman, W. M. (2000) Nat. Biotechnol. 18, 33-37. - PubMed
-
- Davis, S. S. (1997) Trends Biotechnol. 15, 217-224. - PubMed
-
- Anderson, W. F. (1998) Nature 392, 25-30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

