Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration
- PMID: 16051702
- PMCID: PMC1183563
- DOI: 10.1073/pnas.0503386102
Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration
Abstract
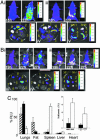
Sequestration of malaria-parasite-infected erythrocytes in the microvasculature of organs is thought to be a significant cause of pathology. Cerebral malaria (CM) is a major complication of Plasmodium falciparum infections, and PfEMP1-mediated sequestration of infected red blood cells has been considered to be the major feature leading to CM-related pathology. We report a system for the real-time in vivo imaging of sequestration using transgenic luciferase-expressing parasites of the rodent malaria parasite Plasmodium berghei. These studies revealed that: (i) as expected, lung tissue is a major site, but, unexpectedly, adipose tissue contributes significantly to sequestration, and (ii) the class II scavenger-receptor CD36 to which PfEMP1 can bind is also the major receptor for P. berghei sequestration, indicating a role for alternative parasite ligands, because orthologues of PfEMP1 are absent from rodent malaria parasites, and, importantly, (iii) cerebral complications still develop in the absence of CD36-mediated sequestration, dissociating parasite sequestration from CM-associated pathology. Real-time in vivo imaging of parasitic processes may be used to evaluate the molecular basis of pathology and develop strategies to prevent pathology.
Figures




Similar articles
-
The machinery underlying malaria parasite virulence is conserved between rodent and human malaria parasites.Nat Commun. 2016 May 26;7:11659. doi: 10.1038/ncomms11659. Nat Commun. 2016. PMID: 27225796 Free PMC article.
-
Reduced CD36-dependent tissue sequestration of Plasmodium-infected erythrocytes is detrimental to malaria parasite growth in vivo.J Exp Med. 2012 Jan 16;209(1):93-107. doi: 10.1084/jem.20110762. Epub 2011 Dec 19. J Exp Med. 2012. PMID: 22184632 Free PMC article.
-
ICAM-1 is a key receptor mediating cytoadherence and pathology in the Plasmodium chabaudi malaria model.Malar J. 2017 May 3;16(1):185. doi: 10.1186/s12936-017-1834-8. Malar J. 2017. PMID: 28468674 Free PMC article.
-
Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria?PLoS Pathog. 2010 Sep 30;6(9):e1001032. doi: 10.1371/journal.ppat.1001032. PLoS Pathog. 2010. PMID: 20941396 Free PMC article. Review.
-
Cerebral malaria pathogenesis: revisiting parasite and host contributions.Future Microbiol. 2012 Feb;7(2):291-302. doi: 10.2217/fmb.11.155. Future Microbiol. 2012. PMID: 22324996 Review.
Cited by
-
Overlapping and distinct roles of CDPK family members in the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium berghei.PLoS Pathog. 2020 Aug 31;16(8):e1008131. doi: 10.1371/journal.ppat.1008131. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866196 Free PMC article.
-
A novel 'gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites.PLoS One. 2011;6(12):e29289. doi: 10.1371/journal.pone.0029289. Epub 2011 Dec 27. PLoS One. 2011. PMID: 22216235 Free PMC article.
-
A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria.Am J Pathol. 2007 Aug;171(2):548-59. doi: 10.2353/ajpath.2007.061033. Epub 2007 Jun 28. Am J Pathol. 2007. PMID: 17600128 Free PMC article.
-
Uric acid is a mediator of the Plasmodium falciparum-induced inflammatory response.PLoS One. 2009;4(4):e5194. doi: 10.1371/journal.pone.0005194. Epub 2009 Apr 17. PLoS One. 2009. PMID: 19381275 Free PMC article.
-
CD36 and Fyn kinase mediate malaria-induced lung endothelial barrier dysfunction in mice infected with Plasmodium berghei.PLoS One. 2013 Aug 15;8(8):e71010. doi: 10.1371/journal.pone.0071010. eCollection 2013. PLoS One. 2013. PMID: 23967147 Free PMC article.
References
-
- Ho, M. & White, N. J. (1999) Am. J. Physiol. 276, C1231-C1242. - PubMed
-
- Sherman, I. W., Eda, S. & Winograd, E. (2003) Microbes Infect. 5, 897-909. - PubMed
-
- Chotivanich, K., Udomsangpetch, R., McGready, R., Proux, S., Newton, P., Pukrittayakamee, S., Looareesuwan, S. & White, N. J. (2002) J. Infect. Dis. 185, 1538-1541. - PubMed
-
- Engwerda, C. R., Beattie, L. & Amante, F. H. (2005) Trends Parasitol. 21, 75-80. - PubMed
-
- Mackintosh, C. L., Beeson, J. G. & Marsh, K. (2004) Trends Parasitol. 20, 597-603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

