Diagnosis and management of paroxysmal nocturnal hemoglobinuria
- PMID: 16051736
- PMCID: PMC1895106
- DOI: 10.1182/blood-2005-04-1717
Diagnosis and management of paroxysmal nocturnal hemoglobinuria
Figures
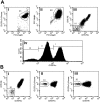
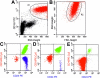

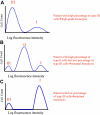
References
-
- Dacie JV, Lewis SM. Paroxysmal nocturnal haemoglobinuria: clinical manifestations, haematology, and nature of the disease. Ser Haematol. 1972;5: 3-23. - PubMed
-
- Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100: 3897-3902. - PubMed
-
- Schubert J, Alvarado M, Uciechowski P, et al. Diagnosis of paroxysmal nocturnal haemoglobinuria using immunophenotyping of peripheral blood cells. Br J Haematol. 1991;79: 487-492. - PubMed
-
- Richards SJ, Rawstron AC, Hillmen P. Application of flow cytometry to the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytometry. 2000;42: 223-233. - PubMed
-
- Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996; 87: 5332-5340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

