EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation
- PMID: 16052208
- PMCID: PMC1356341
- DOI: 10.1038/sj.emboj.7600759
EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation
Abstract
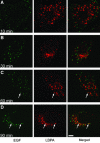
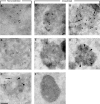
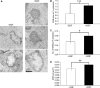
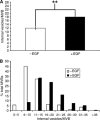
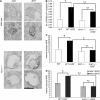
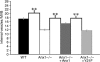
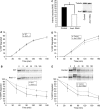
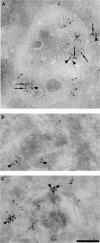
Here we show that EGF and EGF receptor (EGFR) are trafficked through a subpopulation of multivesicular endosomes/bodies (MVBs) that are distinct from morphologically identical vacuoles that label for the late endosomal marker lyso-bisphosphatidic acid (LBPA). EGF stimulation increases both MVB biogenesis and inward vesiculation within EGFR-containing MVBs. Deletion of annexin 1, a substrate of EGFR tyrosine kinase, abolishes the effect of EGF stimulation on inward vesiculation. This phenotype is reversible by transfection with wild-type but not Y21F phosphorylation mutant annexin 1. Deletion of annexin 1 has no effect on EGF-stimulated MVB biogenesis, suggesting that MVB biogenesis and inward vesiculation within MVB are mediated by separate mechanisms. Loss or depletion of annexin 1 has no effect on EGF degradation and causes only a small delay in EGFR degradation, indicating that annexin 1 operates downstream of Hrs- and ESCRT-mediated sorting and is required solely for EGF-stimulated inward vesiculation. Annexin 1 accumulates on internal vesicles of MVB after EGF-stimulated inward vesiculation, suggesting that it may be required for a late stage in inward vesiculation.
Figures

References
-
- Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD (2002a) Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3: 271–282 - PubMed
-
- Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD (2002b) Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 3: 283–289 - PubMed
-
- Babst M, Odorizzi G, Estepa EJ, Emr SD (2000) Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1: 248–258 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

