Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells
- PMID: 16052215
- PMCID: PMC2361561
- DOI: 10.1038/sj.bjc.6602720
Fluvastatin synergistically enhances the antiproliferative effect of gemcitabine in human pancreatic cancer MIAPaCa-2 cells
Abstract
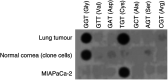
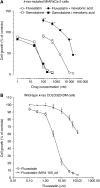
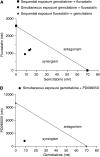
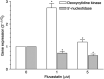
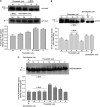
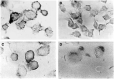
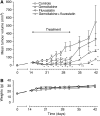
The new combination between the nucleoside analogue gemcitabine and the cholesterol-lowering drug fluvastatin was investigated in vitro and in vivo on the human pancreatic tumour cell line MIAPaCa-2. The present study demonstrates that fluvastatin inhibits proliferation, induces apoptosis in pancreatic cancer cells harbouring a p21ras mutation at codon 12 and synergistically potentiates the cytotoxic effect of gemcitabine. The pharmacologic activities of fluvastatin are prevented by administration of mevalonic acid, suggesting that the shown inhibition of geranyl-geranylation and farnesylation of cellular proteins, including p21rhoA and p21ras, plays a major role in its anticancer effect. Fluvastatin treatment also indirectly inhibits the phosphorylation of p42ERK2/mitogen-activated protein kinase, the cellular effector of ras and other signal transduction peptides. Moreover, fluvastatin administration significantly increases the expression of the deoxycytidine kinase, the enzyme required for the activation of gemcitabine, and simultaneously reduces the 5'-nucleotidase, responsible for deactivation of gemcitabine, suggesting a possible additional role of these enzymes in the enhanced cytotoxic activity of gemcitabine. Finally, a significant in vivo antitumour effect on MIAPaCa-2 xenografts was observed with the simultaneous combination of fluvastatin and gemcitabine, resulting in an almost complete suppression and a marked delay in relapse of tumour growth. In conclusion, the combination of fluvastatin and gemcitabine is an effective cytotoxic, proapoptotic treatment in vitro and in vivo against MIAPaCa-2 cells by a mechanism of action mediated, at least in part, by the inhibition of p21ras and rhoA prenylation. The obtained experimental findings might constitute the basis for a novel translational research in humans.
Figures


References
-
- Abbruzzese JL (2002a) New applications of gemcitabine and future directions in the management of pancreatic cancer. Cancer 95: 941–945 - PubMed
-
- Abbruzzese JL (2002b) Past and present treatment of pancreatic adenocarcinoma: chemotherapy as a standard treatment modality. Semin Oncol 29: 2–8 - PubMed
-
- Agarwal B, Bhendwal S, Halmos B, Moss SF, Ramey WG, Holt PR (1999) Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res 5: 2223–2229 - PubMed
-
- Barton-Burke M (1999) Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs 22: 176–183 - PubMed
-
- Bergman AM, Ruiz vHV, Veerman G, Kuiper CM, Peters GJ (1996) Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 2: 521–530 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

