A systematic review of taxane-containing regimens for metastatic breast cancer
- PMID: 16052223
- PMCID: PMC2361568
- DOI: 10.1038/sj.bjc.6602680
A systematic review of taxane-containing regimens for metastatic breast cancer
Abstract
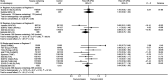
We compared the results of randomised trials comparing taxane-containing chemotherapy regimens with regimens not containing a taxane in women with metastatic breast cancer. The specialised register of the Cochrane Breast Cancer Group was searched in March 2004. Eligibility was assessed and data extracted from eligible studies by two reviewers. Hazard ratios (HR) were derived for time-to-event outcomes, and a fixed-effect model was used for meta-analysis. Tumour response rates were analysed as dichotomous variables. Of 21 eligible trials, 16 had published some results and 12 data on overall survival. An estimated 2621 deaths among 3643 women suggest a significant difference in overall survival in favour of taxane-containing regimens (HR 0.93, 95% confidence interval (CI) 0.86-1.00, P=0.05). The treatment effect on survival was similar if only trials of first-line chemotherapy were included, although not statistically significant. There appeared to be an advantage for taxanes in time to progression (HR 0.92, 95% CI 0.85-0.99, P=0.02) and overall response (odds ratio (OR) 1.34, 95% CI 1.18-1.52, P<0.001). There was significant heterogeneity across the trials (P<0.001), partly because of the varying efficacy of the comparator regimens. Taxane-containing regimens improved overall survival in women with metastatic breast cancer. Taxane-containing regimens are more effective than some, but not all, nontaxane-containing regimens.
Figures




References
-
- Alderson P, Green S, Higgins JPT (eds) (2003) Cochrane Reviewers' Handbook 4.2.2. Chichester, UK, Ltd: John Wiley & Sons, Available at http://www.cochrane.org/resources/handbook/hbook.htm (accessed 31 January 2005)
-
- Bernard-Marty C, Cardoso F, Piccart MJ (2003) Use and abuse of taxanes in the management of metastatic breast cancer. Eur J Cancer 39: 1978–1989 - PubMed
-
- Biganzoli L, Cufer T, Bruning P, Coleman R, Duchateau L, Calvert AH, Gamucci T, Twelves C, Fargeot P, Epelbaum R, Lohrisch C, Piccart MJ (2002) Doxorubicin and paclitaxel vs doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: EORTC 10961. J Clin Oncol 20(14): 3114–3121 - PubMed
-
- Bishop JF, Dewar J, Toner GC, Smith J, Tattersall MH, Olver IN, Ackland S, Kennedy I, Goldstein D, Gurney H, Walpole E, Levi J, Stephenson J, Canetta RL (1999) Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol 17(8): 2355–2364 - PubMed
-
- Bonneterre J, Dieras V, Tubiana-Hulin M, Bougnoux P, Bonneterre M, Delozier T, Culine S, Dohollou N, Suissa J, Samak R (2001) 6 cycles of epirubicin/docetaxel (ET) vs 6 cycles of 5FU epirubicin/cyclophosphamide (FEC) as first line metastatic breast cancer (MBC) treatment. Proceedings of the American Society of Clinical Oncology Abstract 163, Available at http://www.asco.org/ac/1,1003,_12-002636-00_18-0010-00_19-00163,00.asp (accessed 15 September 2004)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

