Regulation of cancer cell motility through actin reorganization
- PMID: 16053508
- PMCID: PMC11159066
- DOI: 10.1111/j.1349-7006.2005.00062.x
Regulation of cancer cell motility through actin reorganization
Abstract
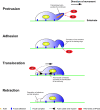
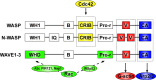
Cell migration is a critical step in tumor invasion and metastasis, and regulation of this process will lead to appropriate therapies for treating cancer. Cancer cells migrate in various ways, according to cell type and degree of differentiation. The different types of cell migration are regulated by different mechanisms. Reorganization of the actin cytoskeleton is the primary mechanism of cell motility and is essential for most types of cell migration. Actin reorganization is regulated by Rho family small GTPases such as Rho, Rac, and Cdc42. These small GTPases transmit extracellular chemotactic signals to downstream effectors. Of these downstream effectors, Wiskott-Aldrich syndrome protein (WASP) family proteins are key regulators of cell migration. Activated WASP family proteins induce the formation of protrusive membrane structures involved in cell migration and degradation of the extracellular matrix. Inhibition of Rho family small GTPase signaling suppresses the migration and invasion of cancer cells. Thus, control of cell migration via the actin cytoskeleton provides the possibility of regulating cancer cell invasion and metastasis.
Figures


References
-
- Ridley AJ, Schwartz MA, Burridge K et al. Cell migration: integrating signals from front to back. Science 2003; 302: 1704–9. - PubMed
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112: 453–65. - PubMed
-
- Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer 2003; 3: 921–30. - PubMed
-
- Kundra V, Escobedo JA, Kazlauskas A et al. Regulation of chemotaxis by the platelet‐derived growth factor receptor‐beta. Nature 1994; 367: 474–6. - PubMed
-
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol 1995; 7: 697–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

