The "soluble" adenylyl cyclase in sperm mediates multiple signaling events required for fertilization
- PMID: 16054031
- PMCID: PMC3082461
- DOI: 10.1016/j.devcel.2005.06.007
The "soluble" adenylyl cyclase in sperm mediates multiple signaling events required for fertilization
Abstract
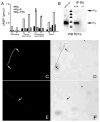
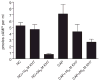
Mammalian fertilization is dependent upon a series of bicarbonate-induced, cAMP-dependent processes sperm undergo as they "capacitate," i.e., acquire the ability to fertilize eggs. Male mice lacking the bicarbonate- and calcium-responsive soluble adenylyl cyclase (sAC), the predominant source of cAMP in male germ cells, are infertile, as the sperm are immotile. Membrane-permeable cAMP analogs are reported to rescue the motility defect, but we now show that these "rescued" null sperm were not hyperactive, displayed flagellar angulation, and remained unable to fertilize eggs in vitro. These deficits uncover a requirement for sAC during spermatogenesis and/or epididymal maturation and reveal limitations inherent in studying sAC function using knockout mice. To circumvent this restriction, we identified a specific sAC inhibitor that allowed temporal control over sAC activity. This inhibitor revealed that capacitation is defined by separable events: induction of protein tyrosine phosphorylation and motility are sAC dependent while acrosomal exocytosis is not dependent on sAC.
Figures



References
-
- Austin CR. The “capacitation” of the mammalian sperm. Nature. 1952;170:326. - PubMed
-
- Baxendale RW, Fraser LR. Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev. 2003;66:181–189. - PubMed
-
- Bellve AR, Millette CF, Bhatnagar YM, O’Brien DA. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977b;25:480–494. - PubMed
-
- Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

