Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation
- PMID: 16054088
- PMCID: PMC3141219
- DOI: 10.1016/j.cmet.2005.05.003
Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation
Abstract
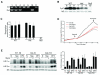
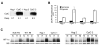
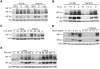
While cellular responses to low oxygen (O(2)) or hypoxia have been studied extensively, the precise identity of mammalian cellular O(2) sensors remains controversial. Using murine embryonic cells lacking cytochrome c, and therefore mitochondrial activity, we show that mitochondrial reactive oxygen species (mtROS) are essential for proper O(2) sensing and subsequent HIF-1 alpha and HIF-2 alpha stabilization at 1.5% O(2). In the absence of this signal, HIF-alpha subunits continue to be degraded. Furthermore, exogenous treatment with H(2)O(2) or severe O(2) deprivation is sufficient to stabilize HIF-alpha even in the absence of cytochrome c and functional mitochondria. These results provide genetic evidence indicating that mtROS act upstream of prolyl hydroxylases in regulating HIF-1 alpha and HIF-2 alpha in this O(2)-sensing pathway.
Figures

References
-
- Agani FH, Pichiule P, Chavez J. Carlos, LaManna JC. Inhibitors of mitochondrial complex I attenuate the accumulation of hypoxia-inducible factor-1 during hypoxia in Hep3B cells. Comp Biochem Physiol A Mol Integr Physiol. 2002a;132:107–109. - PubMed
-
- Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000;275:35863–35867. - PubMed
-
- Agani FH, Puchowicz M, Chavez JC, Pichiule P, LaManna J. Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia. Am J Physiol Cell Physiol. 2002b;283:C178–186. - PubMed
-
- Barros MH, Netto LE, Kowaltowski AJ. H(2)O(2) generation in Saccharomyces cerevisiae respiratory pet mutants: effect of cytochrome c. Free Radic Biol Med. 2003;35:179–188. - PubMed
-
- BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem. 2004;385:249–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

