SNARE-driven, 25-millisecond vesicle fusion in vitro
- PMID: 16055544
- PMCID: PMC1366745
- DOI: 10.1529/biophysj.105.062539
SNARE-driven, 25-millisecond vesicle fusion in vitro
Abstract
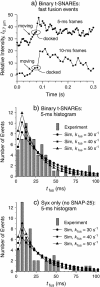
Docking and fusion of single proteoliposomes reconstituted with full-length v-SNAREs (synaptobrevin) into planar lipid bilayers containing binary t-SNAREs (anchored syntaxin associated with SNAP25) was observed in real time by wide-field fluorescence microscopy. This enabled separate measurement of the docking rate k(dock) and the unimolecular fusion rate k(fus). On low t-SNARE-density bilayers at 37 degrees C, docking is efficient: k(dock) = 2.2 x 10(7) M(-1) s(-1), approximately 40% of the estimated diffusion limited rate. Full vesicle fusion is observed as a prompt increase in fluorescence intensity from labeled lipids, immediately followed by outward radial diffusion (D(lipid) = 0.6 microm2 s(-1)); approximately 80% of the docked vesicles fuse promptly as a homogeneous subpopulation with k(fus) = 40 +/- 15 s(-1) (tau(fus) = 25 ms). This is 10(3)-10(4) times faster than previous in vitro fusion assays. Complete lipid mixing occurs in <15 ms. Both the v-SNARE and the t-SNARE are necessary for efficient docking and fast fusion, but Ca2+ is not. Docking and fusion were quantitatively similar on syntaxin-only bilayers lacking SNAP25. At present, in vitro fusion driven by SNARE complexes alone remains approximately 40 times slower than the fastest, submillisecond presynaptic vesicle population response.
Figures



References
-
- Katz, B. 1969. The Release of Neural Transmitter Substances. Charles C. Thomas, Springfield, IL.
-
- Augustine, G. J. 2001. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11:320–326. - PubMed
-
- Sollner, T. H. 2003. Regulated exocytosis and SNARE function (Review). Mol. Membr. Biol. 20:209–220. - PubMed
-
- Bai, J., and E. R. Chapman. 2004. The C2 domains of synaptotagmin—partners in exocytosis. Trends Biochem. Sci. 29:143–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

