Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli
- PMID: 16055670
- PMCID: PMC2715353
- DOI: 10.1165/rcmb.2005-0180OC
Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli
Abstract
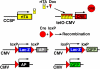
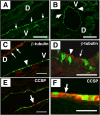
To identify relationships amongst tracheal and alveolar epithelial cells during lung development, we used conditional systems controlled by the rat CCSP and human SFTPC gene promoters to express Cre-recombinase in the developing mouse lung, thereby permanently labeling cells by expression of alkaline phosphatase or green fluorescent protein. When controlled by the rat CCSP promoter, continuous exposure of the fetus to doxycycline caused widespread recombination in conducting airway epithelial cells, including cells of the trachea, bronchi, and bronchioles before birth, and in both conducting and peripheral airways after birth. Neuroepithelial cells, identified by CGRP staining, were never labeled. Recombination and permanent labeling were observed in both ciliated and nonciliated respiratory epithelial cells, demonstrating their derivation from common progenitor cells during lung morphogenesis. Remarkable dorsal-ventral and cephalo-caudal labeling patterns, established before birth, were identified by recombination controlled by the rat CCSP gene promoter. In the trachea, subsets of epithelial cells labeled by the CCSP promoter were organized horizontally along the dorsal-ventral axis of the trachea, where selective labeling of cells juxtaposed to tracheal and bronchial cartilage was observed. In sharp contrast, recombination controlled by the human SFTPC gene promoter identified related cells that were organized in linear patterns along the cephalo-caudal axis of the conducting airways. Conditional expression of Cre-recombinase in the respiratory epithelium provides a useful model for the study of gene expression and function in the mouse respiratory tract and in the lung.
Figures






References
-
- Ten Have-Opbroek AA. Lung development in the mouse embryo. Exp Lung Res 1991;17:111–130. - PubMed
-
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol 1994;166:600–614. - PubMed
-
- Cardoso WV. Molecular regulation of lung development. Annu Rev Physiol 2001;63:471–494. - PubMed
-
- Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis 1983;128:S14–S20. - PubMed
-
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

