Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis
- PMID: 16055687
- PMCID: PMC1183394
- DOI: 10.1104/pp.105.061218
Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis
Abstract
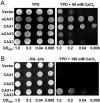
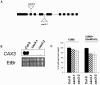
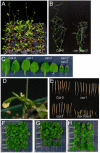
Cation levels within the cytosol are coordinated by a network of transporters. Here, we examine the functional roles of calcium exchanger 1 (CAX1), a vacuolar H+/Ca2+ transporter, and the closely related transporter CAX3. We demonstrate that like CAX1, CAX3 is also localized to the tonoplast. We show that CAX1 is predominately expressed in leaves, while CAX3 is highly expressed in roots. Previously, using a yeast assay, we demonstrated that an N-terminal truncation of CAX1 functions as an H+/Ca2+ transporter. Here, we use the same yeast assay to show that full-length CAX1 and full-length CAX3 can partially, but not fully, suppress the Ca2+ hypersensitive yeast phenotype and coexpression of full-length CAX1 and CAX3 conferred phenotypes not produced when either transporter was expressed individually. In planta, CAX3 null alleles were modestly sensitive to exogenous Ca2+ and also displayed a 22% reduction in vacuolar H+-ATPase activity. cax1/cax3 double mutants displayed a severe reduction in growth, including leaf tip and flower necrosis and pronounced sensitivity to exogenous Ca2+ and other ions. These growth defects were partially suppressed by addition of exogenous Mg2+. The double mutant displayed a 42% decrease in vacuolar H+/Ca2+ transport, and a 47% decrease in H+-ATPase activity. While the ionome of cax1 and cax3 lines were modestly perturbed, the cax1/cax3 lines displayed increased PO4(3-), Mn2+, and Zn2+ and decreased Ca2+ and Mg2+ in shoot tissue. These findings suggest synergistic function of CAX1 and CAX3 in plant growth and nutrient acquisition.
Figures


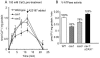

References
-
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Cheng NH, Hirschi KD (2003) Cloning and characterization of CXIP1, a novel PICOT domain-containing Arabidopsis protein that associates with CAX1. J Biol Chem 278: 6503–6509 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

