Subunit contributions to histone methyltransferase activities of fly and worm polycomb group complexes
- PMID: 16055700
- PMCID: PMC1190254
- DOI: 10.1128/MCB.25.16.6857-6868.2005
Subunit contributions to histone methyltransferase activities of fly and worm polycomb group complexes
Abstract
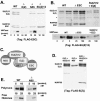
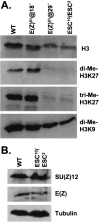
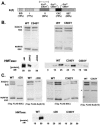
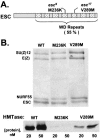
The ESC-E(Z) complex of Drosophila melanogaster Polycomb group (PcG) repressors is a histone H3 methyltransferase (HMTase). This complex silences fly Hox genes, and related HMTases control germ line development in worms, flowering in plants, and X inactivation in mammals. The fly complex contains a catalytic SET domain subunit, E(Z), plus three noncatalytic subunits, SU(Z)12, ESC, and NURF-55. The four-subunit complex is >1,000-fold more active than E(Z) alone. Here we show that ESC and SU(Z)12 play key roles in potentiating E(Z) HMTase activity. We also show that loss of ESC disrupts global methylation of histone H3-lysine 27 in fly embryos. Subunit mutations identify domains required for catalytic activity and/or binding to specific partners. We describe missense mutations in surface loops of ESC, in the CXC domain of E(Z), and in the conserved VEFS domain of SU(Z)12, which each disrupt HMTase activity but preserve complex assembly. Thus, the E(Z) SET domain requires multiple partner inputs to produce active HMTase. We also find that a recombinant worm complex containing the E(Z) homolog, MES-2, has robust HMTase activity, which depends upon both MES-6, an ESC homolog, and MES-3, a pioneer protein. Thus, although the fly and mammalian PcG complexes absolutely require SU(Z)12, the worm complex generates HMTase activity from a distinct partner set.
Figures

References
-
- Bender, L. B., R. Cao, Y. Zhang, and S. Strome. 2004. The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr. Biol. 14:1639-1643. - PubMed
-
- Birve, A., A. K. Sengupta, D. Beuchle, J. Larsson, J. A. Kennison, A. Rasmuson-Lestander, and J. Muller. 2001. Su(z)12, a novel Drosophila Polycomb group gene that is conserved in vertebrates and plants. Development 128:3371-3379. - PubMed
-
- Cao, R., L. Wang, H. Wang, L. Xia, H. Erdjument-Bromage, P. Tempst, R. S. Jones, and Y. Zhang. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298:1039-1043. - PubMed
-
- Cao, R., and Y. Zhang. 2004. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14:155-164. - PubMed
-
- Cao, R., and Y. Zhang. 2004. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol. Cell 15:57-67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
