Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway
- PMID: 16055703
- PMCID: PMC1190270
- DOI: 10.1128/MCB.25.16.6889-6898.2005
Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway
Abstract
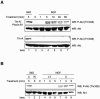
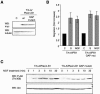
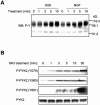
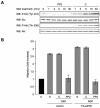
Semaphorins are cell surface and secreted proteins that provide axonal guidance in neuronal tissues and regulate cell motility in many cell types. They act by binding a family of transmembrane receptors known as plexins, which belong to the c-Met family of scatter factor receptors but lack an intrinsic tyrosine kinase domain. Interestingly, we have recently shown that Plexin-B1 is highly expressed in endothelial cells and that its activation by Semaphorin 4D elicits a potent proangiogenic response (J. R. Basile, A. Barac, T. Zhu, K. L. Guan, and J. S. Gutkind, Cancer Res. 64:5212-5224, 2004). In searches for the underlying molecular mechanism, we observed that Semaphorin 4D-stimulated endothelial cell migration requires the activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. Surprisingly, we found that Plexin-B1 stimulates PI3K-Akt through the activation of an intracellular tyrosine kinase cascade that involves the sequential activation of PYK2 and Src. This results in the tyrosine phosphorylation of Plexin-B1, the rapid recruitment of a multimeric signaling complex that includes PYK2, Src, and PI3K to Plexin-B1 and the activation of Akt. These findings suggest that Plexin-B1 may achieve its numerous physiological functions through the direct activation of intracellular tyrosine kinase cascades.
Figures




References
-
- Anonymous. 1999. Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 97:551-552. - PubMed
-
- Avraham, H., S. Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12:123-133. - PubMed
-
- Basile, J. R., A. Barac, T. Zhu, K. L. Guan, and J. S. Gutkind. 2004. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 64:5212-5224. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
