Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks
- PMID: 16055725
- PMCID: PMC1190269
- DOI: 10.1128/MCB.25.16.7158-7169.2005
Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks
Abstract
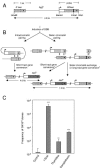
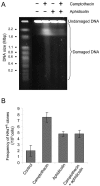
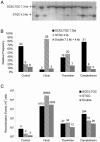
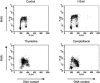
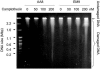
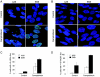
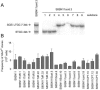
Homologous recombination is vital to repair fatal DNA damage during DNA replication. However, very little is known about the substrates or repair pathways for homologous recombination in mammalian cells. Here, we have compared the recombination products produced spontaneously with those produced following induction of DNA double-strand breaks (DSBs) with the I-SceI restriction endonuclease or after stalling or collapsing replication forks following treatment with thymidine or camptothecin, respectively. We show that each lesion produces different spectra of recombinants, suggesting differential use of homologous recombination pathways in repair of these lesions. The spontaneous spectrum most resembled the spectra produced at collapsed replication forks formed when a replication fork runs into camptothecin-stabilized DNA single-strand breaks (SSBs) within the topoisomerase I cleavage complex. We found that camptothecin-induced DSBs and the resulting recombination repair require replication, showing that a collapsed fork is the substrate for camptothecin-induced recombination. An SSB repair-defective cell line, EM9 with an XRCC1 mutation, has an increased number of spontaneous gammaH2Ax and RAD51 foci, suggesting that endogenous SSBs collapse replication forks, triggering recombination repair. Furthermore, we show that gammaH2Ax, DSBs, and RAD51 foci are synergistically induced in EM9 cells with camptothecin, suggesting that lack of SSB repair in EM9 causes more collapsed forks and more recombination repair. Furthermore, our results suggest that two-ended DSBs are rare substrates for spontaneous homologous recombination in a mammalian fibroblast cell line. Interestingly, all spectra showed evidence of multiple homologous recombination events in 8 to 16% of clones. However, there was no increase in homologous recombination genomewide in these clones nor were the events dependent on each other; rather, we suggest that a first homologous recombination event frequently triggers a second event at the same locus in mammalian cells.
Figures

References
-
- Arnaudeau, C., C. Lundin, and T. Helleday. 2001. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 307:1235-1245. - PubMed
-
- Arnaudeau, C., E. Tenorio Miranda, D. Jenssen, and T. Helleday. 2000. Inhibition of DNA synthesis is a potent mechanism by which cytostatic drugs induce homologous recombination in mammalian cells. Mutat. Res. DNA Repair 461:221-228. - PubMed
-
- Bryant, H. E., N. Schultz, H. D. Thomas, K. M. Parker, D. Flower, E. Lopez, S. Kyle, M. Meuth, N. J. Curtin, and T. Helleday. 2005. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose)polymerase. Nature 434:913-917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
