Signal regulatory protein alpha ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways
- PMID: 16055727
- PMCID: PMC1190262
- DOI: 10.1128/MCB.25.16.7181-7192.2005
Signal regulatory protein alpha ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways
Abstract
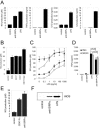
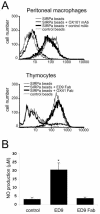
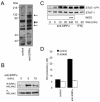
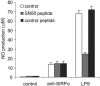
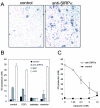
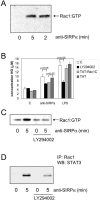
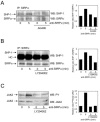
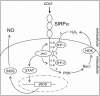
Signal regulatory protein alpha (SIRPalpha) is a glycoprotein receptor that recruits and signals via the tyrosine phosphatases SHP-1 and SHP-2. In macrophages SIRPalpha can negatively regulate the phagocytosis of host cells and the production of tumor necrosis factor alpha. Here we provide evidence that SIRPalpha can also stimulate macrophage activities, in particular the production of nitric oxide (NO) and reactive oxygen species. Ligation of SIRPalpha by antibodies or soluble CD47 triggers inducible nitric oxide synthase expression and production of NO. This was not caused by blocking negative-regulatory SIRPalpha-CD47 interactions. SIRPalpha-induced NO production was prevented by inhibition of the tyrosine kinase JAK2. JAK2 was found to associate with SIRPalpha in macrophages, particularly after SIRPalpha ligation, and SIRPalpha stimulation resulted in JAK2 and STAT1 tyrosine phosphorylation. Furthermore, SIRPalpha-induced NO production required the generation of hydrogen peroxide (H(2)O(2)) by a NADPH oxidase (NOX) and the phosphatidylinositol 3-kinase (PI3-K)-dependent activation of Rac1, an intrinsic NOX component. Finally, SIRPalpha ligation promoted SHP-1 and SHP-2 recruitment, which was both JAK2 and PI3-K dependent. These findings demonstrate that SIRPalpha ligation induces macrophage NO production through the cooperative action of JAK/STAT and PI3-K/Rac1/NOX/H(2)O(2) signaling pathways. Therefore, we propose that SIRPalpha is able to function as an activating receptor.
Figures
References
-
- Adams, S., L. J. van der Laan, E. Vernon-Wilson, C. Renardel de Lavalette, E. A. Dopp, C. D. Dijkstra, D. L. Simmons, and T. K. van den Berg. 1998. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 161:1853-1859. - PubMed
-
- Bright, J. J., C. Natarajan, S. Sriram, and G. Muthian. 2004. Signaling through JAK2-STAT5 pathway is essential for IL-3-induced activation of microglia. Glia 45:188-196. - PubMed
-
- Brooke, G., J. D. Holbrook, M. H. Brown, and A. N. Barclay. 2004. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 173:2562-2570. - PubMed
-
- de Vries, H. E., J. J. Hendriks, H. Honing, C. R. De Lavalette, S. M. van der Pol, E. Hooijberg, C. D. Dijkstra, and T. K. van den Berg. 2002. Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J. Immunol. 168:5832-5839. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
