Exploiting the keratin 17 gene promoter to visualize live cells in epithelial appendages of mice
- PMID: 16055733
- PMCID: PMC1190235
- DOI: 10.1128/MCB.25.16.7249-7259.2005
Exploiting the keratin 17 gene promoter to visualize live cells in epithelial appendages of mice
Abstract
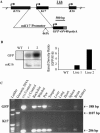
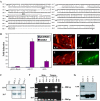
Keratin genes afford, given their large number (>50) and differential regulation, a unique opportunity to study the mechanisms underlying specification and differentiation in epithelia of higher metazoans. Moreover, the small size and regulation in cis of many keratin genes enable the use of their regulatory sequence to achieve targeted gene expression in mice. Here we show that 2 kilobases of 5' upstream region from the mouse keratin 17 gene (mK17) confers expression of green fluorescent protein (GFP) in major epithelial appendages of transgenic mice. Like that of mK17, onset of [mK17 5']-GFP reporter expression coincides with the appearance of ectoderm-derived epithelial appendages during embryonic development. In adult mice, [mK17 5']-GFP is appropriately regulated within hair, nail, glands, and oral papilla. Tracking of GFP fluorescence allows for the visualization of growth cycle-related changes in hair follicles, and the defects engendered by the hairless mutation, in live skin tissue. Deletion of an internal 48-bp interval, which encompasses a Gli-responsive element, from this promoter results in loss of GFP fluorescence in most appendages in vivo, suggesting that sonic hedgehog participates in K17 regulation. The compact mK17 gene promoter provides a novel tool for appendage-preferred gene expression and manipulation in transgenic mice.
Figures



References
-
- Andl, T., S. T. Reddy, T. Gaddapara, and S. E. Millar. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2:643-653. - PubMed
-
- Bernot, K. M., P. A. Coulombe, and K. M. McGowan. 2002. Keratin 16 expression defines a subset of epithelial cells during skin morphogenesis and the hair cycle. J. Investig. Dermatol. 119:1137-1149. - PubMed
-
- Brembeck, F. H., and A. K. Rustgi. 2000. The tissue-dependent keratin 19 gene transcription is regulated by GKLF/KLF4 and Sp1. J. Biol. Chem. 275:28230-28239. - PubMed
-
- Bruen, K. J., C. A. Campbell, W. G. Schooler, S. deSerres, B. A. Cairns, C. S. Hultman, A. A. Meyer, and S. H. Randell. 2004. Real-time monitoring of keratin 5 expression during burn re-epithelialization. J. Surg. Res. 120:12-20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
