Use-dependent inhibition of the skeletal muscle ryanodine receptor by the suramin analogue NF676
- PMID: 16056233
- PMCID: PMC1751178
- DOI: 10.1038/sj.bjp.0706359
Use-dependent inhibition of the skeletal muscle ryanodine receptor by the suramin analogue NF676
Abstract
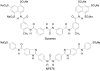
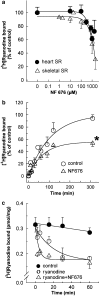
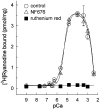
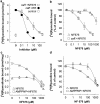
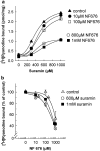
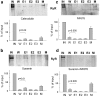
The skeletal muscle Ca2+ release channel, the ryanodine receptor, is activated by the trypanocidal drug suramin via the calmodulin-binding site. As calmodulin activates and inhibits the ryanodine receptor depending on whether Ca2+ is absent or present, suramin analogues were screened for inhibition of the ryanodine receptor. Up to 300 microM, the novel suramin analogue, 4,4'-(carbonyl-bis(imino-4,1-phenylene-(2,5-benzimidazolylene)carbonylimino))-bis-benzenesulfonic acid disodium salt (NF676) was not able to significantly inhibit the basal [3H]ryanodine binding. However, kinetic analysis of the high affinity [3H]ryanodine binding elucidates a time-dependent increment of inhibition by NF676, which is indicative for an open channel blocker. Moreover, the ryanodine receptor was much more sensitive towards inhibition by NF676 when preactivated with caffeine or the nonhydrolysable ATP analogue, adenylyl-imidodiphosphate. Nonetheless, the suramin activated ryanodine receptor was not susceptible towards high-affinity NF676 inhibition, indicating an allosteric hindrance between the binding sites of suramin and NF676. In the line of this finding, NF676 per se was not capable to elute the purified ryanodine receptor from a calmodulin-Sepharose, but it prevented the elution by suramin. Other than suramin, NF676 did not inhibit the Ca2+ ATPase of the sarcoplasmic reticulum. However, suramin-induced Ca2+ release from sarcoplasmic reticulum was completely abrogated by preincubation with NF676. Taken together, we conclude from these data that NF676 represents a novel lead compound as a potent use-dependent blocker of the skeletal muscle ryanodine receptor via an allosteric interaction with the suramin-binding site.
Figures

Similar articles
-
Suramin and suramin analogs activate skeletal muscle ryanodine receptor via a calmodulin binding site.Mol Pharmacol. 1999 Mar;55(3):462-72. Mol Pharmacol. 1999. PMID: 10051529
-
Activation of the skeletal muscle ryanodine receptor by suramin and suramin analogs.Mol Pharmacol. 1996 Dec;50(6):1443-53. Mol Pharmacol. 1996. PMID: 8967964
-
Short- and long-term functional alterations of the skeletal muscle calcium release channel (Ryanodine receptor) by Suramin: apparent dissociation of single channel current recording and [3H]ryanodine binding.Mol Pharmacol. 2001 Mar;59(3):543-56. doi: 10.1124/mol.59.3.543. Mol Pharmacol. 2001. PMID: 11179450
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
-
RyR1 modulation by oxidation and calmodulin.Antioxid Redox Signal. 2000 Spring;2(1):41-5. doi: 10.1089/ars.2000.2.1-41. Antioxid Redox Signal. 2000. PMID: 11232598 Review.
Cited by
-
The steroid hormone 20-hydroxyecdysone via nongenomic pathway activates Ca2+/calmodulin-dependent protein kinase II to regulate gene expression.J Biol Chem. 2015 Mar 27;290(13):8469-81. doi: 10.1074/jbc.M114.622696. Epub 2015 Feb 10. J Biol Chem. 2015. PMID: 25670853 Free PMC article.
-
Purinergic signalling in the musculoskeletal system.Purinergic Signal. 2013 Dec;9(4):541-72. doi: 10.1007/s11302-013-9381-4. Epub 2013 Aug 14. Purinergic Signal. 2013. PMID: 23943493 Free PMC article. Review.
-
G-protein-coupled receptor kinase 2 terminates G-protein-coupled receptor function in steroid hormone 20-hydroxyecdysone signaling.Sci Rep. 2016 Jul 14;6:29205. doi: 10.1038/srep29205. Sci Rep. 2016. PMID: 27412951 Free PMC article.
-
NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10678-83. doi: 10.1073/pnas.0809997106. Epub 2009 Jun 16. Proc Natl Acad Sci U S A. 2009. PMID: 19541638 Free PMC article.
-
Potentiation of regulatory volume decrease by a p2-like receptor and arachidonic acid in american alligator erythrocytes.J Membr Biol. 2011 Jul;242(2):75-87. doi: 10.1007/s00232-011-9377-3. Epub 2011 Jul 5. J Membr Biol. 2011. PMID: 21728043
References
-
- BURATTI R., PRESTIPINOM G., MENEGAZZIM P., TREVES S., ZORZATO F. Calcium dependent activation of skeletal muscle Ca2+ release channel (ryanodine receptor) by calmodulin. Biochem. Biophys. Res. Commun. 1995;213:1082–1090. - PubMed
-
- CHU A., DIAZ-MUNOZ M., HAWKES M.J., BRUSH K., HAMILTON S.L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol. Pharmacol. 1990;37:735–741. - PubMed
-
- EMMICK J.T., KWON S., BIDASEE K.R., BESCH K.T., BESCH H.R. Dual effect of suramin on calcium fluxes across sarcoplasmic reticulum vesicle membranes. J. Pharmacol. Exp. Ther. 1994;269:717–724. - PubMed
-
- FRANZINI-ARMSTRONG C., PROTASI F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. - PubMed
-
- FREISSMUTH M., BOEHM S., BEINDL W., NICKEL P., IJZERMAN A.P., HOHENEGGER M., NANOFF C. Suramin analogues as subtype-selective G protein inhibitors. Mol. Pharmacol. 1996;49:602–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

