Effect of olvanil and anandamide on vagal C-fiber subtypes in guinea pig lung
- PMID: 16056239
- PMCID: PMC1751189
- DOI: 10.1038/sj.bjp.0706339
Effect of olvanil and anandamide on vagal C-fiber subtypes in guinea pig lung
Abstract
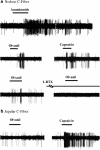
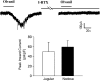
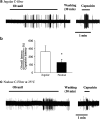
Certain fatty acid amides such as anandamide (AEA) and olvanil are agonists for the transient receptor potential, vanilloid-1 (TRPV1) receptor, but have been found to activate TRPV1-containing C-fibers in some tissues but not others. We used extracellular recording and whole-cell patch clamp techniques to investigate the effect of olvanil and AEA on different types of vagal C-fibers innervating the same tissue, namely jugular and nodose vagal C-fibers in guinea pig lungs. A 30 s exposure to AEA and olvanil caused action potential discharge in all nodose C-fiber innervating lung but failed to activate jugular C-fibers innervating lung and airways. The activation of nodose C-fibers was blocked by the TRPV1 antagonist iodo-resiniferatoxin. In whole-cell patch clamp recordings of dissociated nodose and jugular capsaicin-sensitive neurons labeled from lungs and airways, olvanil induced large TRPV1-dependent inward currents in cell bodies of both nodose and jugular ganglion neurons. Prolonged exposure (up to 5 min) to olvanil caused action potential discharge in jugular C-fiber innervating lung but the onset latency was four times longer in jugular than in nodose C-fibers. The onsets of capsaicin response in nodose and jugular C-fibers were not different. Decreasing the tissue temperature to 25 degrees C increased the onset latency of olvanil-induced activation of nodose C-fibers 2-3-fold, but did not effect the latency of the capsaicin response. Capsaicin, olvanil, and AEA stimulate jugular C-fibers leading to tachykinergic contractions of isolated bronchi. The time to reach half-maximum is more than four times longer for olvanil and AEA, as compared to capsaicin in evoking contractions. We conclude that brief exposure to certain fatty acid amides, such as AEA and olvanil activate nodose but not jugular C-fiber terminals in the lungs. We hypothesize that this is because the nodose C-fiber terminals are equipped with a temperature-dependent mechanism for effectively and rapidly transporting the TRPV1 agonists so that they gain access to the intracellular binding sites on TRPV1. This transport mechanism may be differently expressed in two distinct subtypes of pulmonary C-fiber terminals innervating the same tissue.
Figures

Similar articles
-
Subtypes of vagal afferent C-fibres in guinea-pig lungs.J Physiol. 2004 May 1;556(Pt 3):905-17. doi: 10.1113/jphysiol.2003.060079. Epub 2004 Feb 20. J Physiol. 2004. PMID: 14978204 Free PMC article.
-
Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs.Pulm Pharmacol Ther. 2005;18(4):269-76. doi: 10.1016/j.pupt.2004.12.010. Pulm Pharmacol Ther. 2005. PMID: 15777609
-
Effects of acid on vagal nociceptive afferent subtypes in guinea pig esophagus.Am J Physiol Gastrointest Liver Physiol. 2014 Aug 15;307(4):G471-8. doi: 10.1152/ajpgi.00156.2014. Epub 2014 Jul 3. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24994852 Free PMC article.
-
Functional morphology and physiological properties of bronchopulmonary C-fiber afferents.Anat Rec A Discov Mol Cell Evol Biol. 2003 Jan;270(1):17-24. doi: 10.1002/ar.a.10005. Anat Rec A Discov Mol Cell Evol Biol. 2003. PMID: 12494486 Review.
-
Interaction between TRPA1 and TRPV1: Synergy on pulmonary sensory nerves.Pulm Pharmacol Ther. 2015 Dec;35:87-93. doi: 10.1016/j.pupt.2015.08.003. Epub 2015 Aug 14. Pulm Pharmacol Ther. 2015. PMID: 26283426 Free PMC article. Review.
Cited by
-
Anti-nociceptive and desensitizing effects of olvanil on capsaicin-induced thermal hyperalgesia in the rat.BMC Pharmacol Toxicol. 2016 Jul 21;17(1):31. doi: 10.1186/s40360-016-0074-9. BMC Pharmacol Toxicol. 2016. PMID: 27439609 Free PMC article.
-
Sensory nerves and airway irritability.Handb Exp Pharmacol. 2009;194(194):139-83. doi: 10.1007/978-3-540-79090-7_5. Handb Exp Pharmacol. 2009. PMID: 19655107 Free PMC article. Review.
-
Excitation of cutaneous C nociceptors by intraplantar administration of anandamide.Brain Res. 2009 May 1;1268:38-47. doi: 10.1016/j.brainres.2009.02.061. Epub 2009 Mar 10. Brain Res. 2009. PMID: 19285051 Free PMC article.
-
Cannabinoid Analgesia in Postoperative Pain Management: From Molecular Mechanisms to Clinical Reality.Int J Mol Sci. 2024 Jun 6;25(11):6268. doi: 10.3390/ijms25116268. Int J Mol Sci. 2024. PMID: 38892456 Free PMC article. Review.
-
Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia.Trends Pharmacol Sci. 2009 Feb;30(2):79-84. doi: 10.1016/j.tips.2008.10.008. Epub 2008 Dec 11. Trends Pharmacol Sci. 2009. PMID: 19070372 Free PMC article. Review.
References
-
- ANDERSSON D.A., ADNER M., HOGESTATT E.D., ZYGMUNT P.M. Mechanisms underlying tissue selectivity of anandamide and other vanilloid receptor agonists. Mol. Pharmacol. 2002;62:705–713. - PubMed
-
- APPENDINO G., DE PETROCELLIS L., TREVISANI M., MINASSI A., DADDARIO N., MORIELLO A.S., GAZZIERI D., LIGRESTI A., CAMPI B., FONTANA G., PINNA C., GEPPETTI P., DI MARZO V. Development of the first ultra-potent ‘capsaicinoid' agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J. Pharmacol. Exp. Ther. 2005;312:561–570. - PubMed
-
- BELTRAMO M., PIOMELLI D. Anandamide transport inhibition by the vanilloid agonist olvanil. Eur. J. Pharmacol. 1999;364:75–78. - PubMed
-
- CHUAYCHOO B., LEE M.-G., KOLLARIK M., UNDEM B.J. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm. Pharmacol. Ther. 2005;18:269–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

