Early biomarkers of psychosis
- PMID: 16060593
- PMCID: PMC3181722
- DOI: 10.31887/DCNS.2005.7.1/frreedman
Early biomarkers of psychosis
Abstract
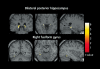
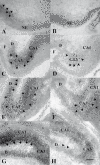
Biological traits that are predictive of the later development of psychosis have not yet been identified. The complex, multidetermined nature of schizophrenia and other psychoses makes it unlikely that any single biomarker will be both sensitive and specific enough to unambiguously identify individuals who will later become psychotic. However, current genetic research has begun to identify genes associated with schizophrenia, some of which have phenotypes that appear early in life. While these phenotypes have low predictive power for identifying individuals who will become psychotic, they do serve as biomarkers for pathophysiological processes that can become the targets of prevention strategies. Examples are given from work on the role of the alpha(T)nicotinic receptor and its gene CHRNA7 on chromosome 15 in the neurobiology and genetic transmission of schizophrenia.
Aun no se han identificado los rasgos biológicos que predicen el desarrollo posterior de una psicosis. La naturaleza compleja y multideterminada de la esquizofrenia y de otras psicosis hace poco probable que algún biomarcador aislado sea lo suficientemente sensible y aspecífico para identificar con certeza sujetos que más tarde llegarán a ser psicóticos. Sin embargo, la investigación genética actual ha comenzado a identificar genes que se asocian con la esquizofrenia, algunos de los cuales tienen fenotipos qua aparacen precozmente en la vida. Aunque estos fenotipos tienen un bajo podar predictor para identificar individuos qua llegarán a ser psicóticos, ellos sirven como biomarcadores de procesos fisiopatológicos que pueden llegar a ser los blancos da las estrategias da prevención. Ejemplos de esto provienen del trabajo acerca del papel del receptor nicotínico α7 y su gen CHRNA 7 en el cromosoma 15 en la neurobiología y en la transmisión genética de la esquizofrenia.
Les particularités biologiques prédictives du développement ultérieur d'une psychose n'ont pas encore été identifiées. La nature complexe, multi-factorielle de la schizophrénie et d'autres psychoses rend peu probable qu'un seul biomarqueur soit a la fois suffisamment sensible et spécifique pour permettre d'identifier sans ambiguïté les individus qui deviendront psychotiques. Cependant, la recherche génétique actuelle a commence a identifier des gènes associes a la schizophrénie, certains ayant des phénotypes qui apparaissent précocement dans le cours de la vie. Alors que ces phénotypes ont un faible pouvoir prédictif pour identifier les individus qui développeront une psychose, ils servent de biomarqueurs pour les processus physiopathologiques pouvant devenir les cibles des stratégies de prévention. Des exemples sont fournis par un travail sur le rôle du récepteur α7-nicotinique et de son gène CHRNA7 situe sur le chromosome 15, en neurobiologie et dans la transmission génétique de la schizophrénie.
Figures





References
-
- Freedman R. N Engl J Med. 2003;349:1738–1749. - PubMed
-
- Freedman R., Leonard S., Olincy A., et al. Evidence for the multigenic inheritance of schizophrenia. Am J Med Genet. 2001;105:794–800. - PubMed
-
- Pulver AE., Lasseter VK., Kasch L., et al. Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet. 1995;60:252–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
