The limited role of NH2-terminal c-Jun phosphorylation in neuronal apoptosis: identification of the nuclear pore complex as a potential target of the JNK pathway
- PMID: 16061693
- PMCID: PMC2171472
- DOI: 10.1083/jcb.200501138
The limited role of NH2-terminal c-Jun phosphorylation in neuronal apoptosis: identification of the nuclear pore complex as a potential target of the JNK pathway
Abstract
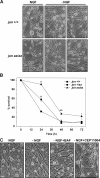
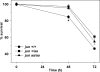
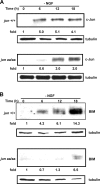
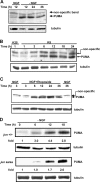
c-Jun is induced in many neuronal death paradigms. A critical step in c-Jun regulation involves phosphorylation of Ser63/Ser73 located in the NH2-terminal transactivation domain. To determine the importance of this phosphorylation for neuronal apoptosis, we analyzed the sympathetic neurons of mice carrying a mutant c-Jun gene that lacks Ser63/Ser73 phosphorylation sites (jun aa). Trophic factor-deprivation or DNA damage-induced death was significantly delayed in jun aa/aa neurons. Neuronal c-Jun induction was only partially inhibited, demonstrating that phosphorylation of Ser63/73 is not required for c-Jun activation. The inductions of proapoptotic BH3-only proteins, Bim and PUMA/Bbc3, were delayed during neuronal apoptosis in mutant neurons. These results demonstrate that NH2-terminal c-Jun phosphorylation is important, but not necessary, for the induction of proapoptotic genes and neuronal apoptosis. Thus, additional JNK substrates may be critical for neuronal death. As potential mediators, we identified additional nuclear MLK/JNK substrates, including Nup214 subunit of the nuclear pore complex.
Figures




References
-
- Angel, P., K. Hattori, T. Smeal, and M. Karin. 1988. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 55:875–885. - PubMed
-
- Behrens, A., M. Sibilia, and E.F. Wagner. 1999. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21:326–329. - PubMed
-
- Besirli, C.G., and E.M. Johnson Jr. 2003. JNK-independent activation of c-Jun during neuronal apoptosis induced by multiple DNA-damaging agents. J. Biol. Chem. 278:22357–22366. - PubMed
-
- Besirli, C.G., T.L. Deckwerth, R.J. Crowder, R.S. Freeman, and E.M. Johnson Jr. 2003. Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ. 10:1045–1058. - PubMed
-
- Coffey, E.T., G. Smiciene, V. Hongisto, J. Cao, S. Brecht, T. Herdegen, and M.J. Courtney. 2002. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 22:4335–4345. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

