Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies
- PMID: 16061794
- PMCID: PMC1183604
- DOI: 10.1073/pnas.0505321102
Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies
Abstract
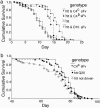
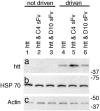
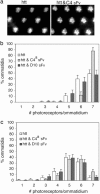
Misfolded neuronal proteins have been identified in a number of neurodegenerative disorders and have been implicated in the pathogenesis of diseases that include Alzheimer's disease, Parkinson's disease, prion-based dementia, Huntington's disease (HD), and other polyglutamine diseases. Although underlying mechanisms remain the subject of ongoing research, it is clear that aberrant processing, protein degradation, and aggregate formation or spurious protein association of the abnormal neuronal proteins may be critical factors in disease progression. Recent work in these diseases has demonstrated in vitro that specific engineered antibody species, peptides, or other general agents may suppress the formation of aggregates. We have modified an approach with intracellularly expressed single-chain Fv (sFv) antibodies (intrabodies) that bind with unique HD protein epitopes. In cell and tissue culture models of HD, anti-N-terminal huntingtin intrabodies (C4 sFv) reduce aggregation and cellular toxicity. Here, we present the crucial experiment of intrabody-mediated in vivo suppression of neuropathology, using a Drosophila model of HD. In the presence of the C4 sFv intrabody, the proportion of HD flies surviving to adulthood increases from 23% to 100%, and the mean and maximum lifespan of adult HD flies is significantly prolonged. Neurodegeneration and formation of visible huntingtin aggregates are slowed. We conclude from this investigation that engineered intrabodies are a potential new class of therapeutic agents for the treatment of neurodegenerative diseases. They may also serve as tools for drug discovery and validation of sites on mutant neuronal proteins that could be exploited for rational drug design.
Figures
Similar articles
-
Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease.Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4764-9. doi: 10.1073/pnas.071058398. Proc Natl Acad Sci U S A. 2001. PMID: 11296304 Free PMC article.
-
A human single-chain Fv intrabody preferentially targets amino-terminal Huntingtin's fragments in striatal models of Huntington's disease.Neurobiol Dis. 2005 Jun-Jul;19(1-2):47-56. doi: 10.1016/j.nbd.2004.11.003. Neurobiol Dis. 2005. PMID: 15837560
-
A single-chain Fv intrabody provides functional protection against the effects of mutant protein in an organotypic slice culture model of Huntington's disease.Brain Res Mol Brain Res. 2004 Feb 5;121(1-2):141-5. doi: 10.1016/j.molbrainres.2003.11.011. Brain Res Mol Brain Res. 2004. PMID: 14969746
-
Intrabody applications in neurological disorders: progress and future prospects.Mol Ther. 2005 Sep;12(3):394-401. doi: 10.1016/j.ymthe.2005.04.003. Mol Ther. 2005. PMID: 15964243 Review.
-
Intrabodies as neuroprotective therapeutics.Neurotherapeutics. 2013 Jul;10(3):447-58. doi: 10.1007/s13311-013-0193-6. Neurotherapeutics. 2013. PMID: 23649691 Free PMC article. Review.
Cited by
-
Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity.J Mol Biol. 2007 May 11;368(4):1132-44. doi: 10.1016/j.jmb.2007.02.089. Epub 2007 Mar 7. J Mol Biol. 2007. PMID: 17391701 Free PMC article.
-
Engineered Extracellular Vesicles/Exosomes as a New Tool against Neurodegenerative Diseases.Pharmaceutics. 2020 Jun 9;12(6):529. doi: 10.3390/pharmaceutics12060529. Pharmaceutics. 2020. PMID: 32526949 Free PMC article. Review.
-
Grape-seed polyphenolic extract improves the eye phenotype in a Drosophila model of tauopathy.Int J Alzheimers Dis. 2010 Aug 24;2010:576357. doi: 10.4061/2010/576357. Int J Alzheimers Dis. 2010. PMID: 20871666 Free PMC article.
-
Screening of therapeutic strategies for Huntington's disease in YAC128 transgenic mice.CNS Neurosci Ther. 2012 Jan;18(1):77-86. doi: 10.1111/j.1755-5949.2011.00246.x. CNS Neurosci Ther. 2012. PMID: 21501423 Free PMC article. Review.
-
Palmitoyl-protein thioesterase 1 deficiency in Drosophila melanogaster causes accumulation of abnormal storage material and reduced life span.Genetics. 2006 Apr;172(4):2379-90. doi: 10.1534/genetics.105.053306. Epub 2006 Feb 1. Genetics. 2006. PMID: 16452138 Free PMC article.
References
-
- The Huntington's Disease Collaborative Research Group (1993) Cell 72, 971-983. - PubMed
-
- Bossy-Wetzel, E., Schwarzenbacher, R. & Lipton, S. A. (2004) Nat. Med. 10, Suppl., S2-S9. - PubMed
-
- Ross, C. A. & Poirier, M. A. (2004) Nat. Med. 10, Suppl. 1, S10-S17. - PubMed
-
- DiFiglia, M., Sapp, E., Chase, K. O., Davies, S. W., Bates, G. P., Vonsattel, J. P. & Aronin, N. (1997) Science 277, 1990-1993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

