Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction
- PMID: 16061805
- PMCID: PMC1183573
- DOI: 10.1073/pnas.0504388102
Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction
Abstract
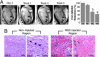
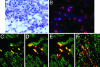
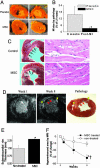
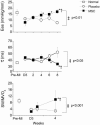
Although clinical trials of autologous whole bone marrow for cardiac repair demonstrate promising results, many practical and mechanistic issues regarding this therapy remain highly controversial. Here, we report the results of a randomized study of bone-marrow-derived mesenchymal stem cells, administered to pigs, which offer several new insights regarding cellular cardiomyoplasty. First, cells were safely injected by using a percutaneous-injection catheter 3 d after myocardial infarction. Second, cellular transplantation resulted in long-term engraftment, profound reduction in scar formation, and near-normalization of cardiac function. Third, transplanted cells were pre-prepared from an allogeneic donor and were not rejected, a major practical advance for widespread application of this therapy. Together, these findings demonstrate that the direct injection of cellular grafts into damaged myocardium is safe and effective in the perii-nfarct period. The direct delivery of cells to necrotic myocardium offers a valuable alternative to intracoronary cell injections, and the use of allogeneic mesenchymal stem cells provides a valuable strategy for cardiac regenerative therapy that avoids the need for preparing autologous cells from the recipient.
Figures

References
-
- Schachinger, V., Assmus, B., Britten, M. B., Honold, J., Lehmann, R., Teupe, C., Abolmaali, N. D., Vogl, T. J., Hofmann, W. K., Martin, H., et al. (2004) J. Am. Coll. Cardiol. 44, 1690-1699. - PubMed
-
- Britten, M. B., Abolmaali, N. D., Assmus, B., Lehmann, R., Honold, J., Schmitt, J., Vogl, T. J., Martin, H., Schachinger, V., Dimmeler, S., et al. (2003) Circulation 108, 2212-2218. - PubMed
-
- Wollert, K. C., Meyer, G. P., Lotz, J., Ringes-Lichtenberg, S., Lippolt, P., Breidenbach, C., Fichtner, S., Korte, T., Hornig, B., Messinger, D., et al. (2004) Lancet 364, 141-148. - PubMed
-
- Assmus, B., Schachinger, V., Teupe, C., Britten, M., Lehmann, R., Dobert, N., Grunwald, F., Aicher, A., Urbich, C., Martin, H., et al. (2002) Circulation 106, 3009-3017. - PubMed
-
- Strauer, B. E., Brehm, M., Zeus, T., Kostering, M., Hernandez, A., Sorg, R. V., Kogler, G. & Wernet, P. (2002) Circulation 106, 1913-1918. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

