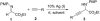Catalytic asymmetric alkynylation of alpha-imino ester: a versatile approach to optically active unnatural alpha-amino acid derivatives
- PMID: 16061808
- PMCID: PMC1183550
- DOI: 10.1073/pnas.0502105102
Catalytic asymmetric alkynylation of alpha-imino ester: a versatile approach to optically active unnatural alpha-amino acid derivatives
Abstract
The catalytic asymmetric introduction of alkynyl functionality to alpha-amino acid derivatives was realized by the direct addition of terminal alkynes to alpha-imino ester in the presence of chiral Cu(I) complex under mild reaction conditions. Owing to the rich chemistry to which alkyne can be subjected, the present system provides a remarkably versatile tool for the construction of optically active alpha-amino acid derivatives. Good yields and enantiomeric excess values were achieved with an array of terminal alkynes and challenging, biologically active, unnatural alpha-amino acid derivatives could be conveniently obtained.
Figures
References
-
- Barrett, G. C. (1985) Chemistry and Biochemistry of the Amino Acids (Chapman & Hall, London).
-
- Williams, R. M. (1989) Synthesis of Optically Active α-Amino Acids (Pergamon, Oxford).
-
- Duthaler, R. O. (1994) Tetrahedron 50, 1539–1650.
-
- Hegedus, L. S. (1995) Acc. Chem. Res. 28, 299–305.
-
- Chin, J. W., Cropp, T. A., Anderson, J. C., Mukherji, M., Zhang, Z. & Schultz, P. G. (2003) Science 301, 964–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources