B7-DC cross-linking restores antigen uptake and augments antigen-presenting cell function by matured dendritic cells
- PMID: 16061819
- PMCID: PMC1183546
- DOI: 10.1073/pnas.0501420102
B7-DC cross-linking restores antigen uptake and augments antigen-presenting cell function by matured dendritic cells
Retraction in
-
Retraction for Radhakrishnan et al., B7-DC cross-linking restores antigen uptake and augments antigen-presenting cell function by matured dendritic cells.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8498. doi: 10.1073/pnas.1003564107. Epub 2010 Apr 7. Proc Natl Acad Sci U S A. 2010. PMID: 20375280 Free PMC article. No abstract available.
Abstract
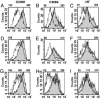
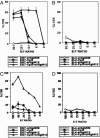
Dendritic cells (DCs) are classified in two states: immature DCs (iDCs), which perform sentinel functions, sampling for antigen and danger signals, and mature DCs (mDCs), which exhibit enhanced antigen-presenting functions but are no longer capable of acquiring antigen. We now describe DCs with a different activation phenotype: cells having the strong antigen-presenting functions of mDCs and the antigen-acquiring functions of iDCs. We have described an antibody that binds the costimulatory molecule B7-DC and activates DCs. The resulting phenotype is distinct from iDCs or mDCs matured by using Toll-like receptor (TLR) agonists. Ability to take up antigen increases, while expression of B71/2 costimulatory and MHC molecules remains unchanged. DCs matured with TLR agonists and then superactivated through B7-DC exhibit a previously unrecognized phenotype. These DCs recover the ability to take up antigen, which is normally lost after treatment with TLR-3 and TLR-9 agonists, yet retain the high expression of costimulatory and MHC molecules and strong antigen-presenting functions of mDCs. Immunization using TLR agonists and B7-DC XAb (cross-linking antibody) together as adjuvant resulted in substantially increased cytolytic T cell responses, particularly when minimal peptide antigens were used. By stimulating DCs with two distinct activation signals, a previously unrecognized phenotype exhibiting augmented antigen-presenting functions was obtained.
Figures



References
-
- Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y.-J., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767-811. - PubMed
-
- Heath, W. R., Belz, G. T., Behrens, G. M., Smith, C. M., Forehan, S. P., Parish, I. A., Davey, G. M., Wilson, N. S., Carbone, F. R. & Villadangos, J. A. (2004) Immunol. Rev. 199, 9-26. - PubMed
-
- Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621-667. - PubMed
-
- Bhardwaj, N., Bender, A., Gonzalez, N., Bui, L. K., Garrett, M. C. & Steinman, R. M. (1995) Adv. Exp. Med. Biol. 378, 375-379. - PubMed
-
- Latchman, Y., Wood, C. R., Chernova, T., Chaudhary, D., Borde, M., Chernova, I., Iwai, Y., Long, A. J., Brown, J. A., Nunes, R. et al. (2001) Nat. Immunol. 2, 261-268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

