Muscle channelopathies and critical points in functional and genetic studies
- PMID: 16075040
- PMCID: PMC1180551
- DOI: 10.1172/JCI25525
Muscle channelopathies and critical points in functional and genetic studies
Abstract
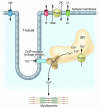
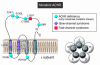
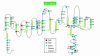
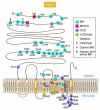
Muscle channelopathies are caused by mutations in ion channel genes, by antibodies directed against ion channel proteins, or by changes of cell homeostasis leading to aberrant splicing of ion channel RNA or to disturbances of modification and localization of channel proteins. As ion channels constitute one of the only protein families that allow functional examination on the molecular level, expression studies of putative mutations have become standard in confirming that the mutations cause disease. Functional changes may not necessarily prove disease causality of a putative mutation but could be brought about by a polymorphism instead. These problems are addressed, and a more critical evaluation of the underlying genetic data is proposed.
Figures


References
-
- Hoffman EP, Lehmann-Horn F, Rüdel R. Overexcited or inactive: ion channels in muscle diseases. Cell. 1995;80:681–686. - PubMed
-
- Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol. Rev. 1999;79:1317–1371. - PubMed
-
- Vincent A, Mills K. Genetic and antibody-mediated channelopathies at the neuromuscular junction. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999;50:250–258. - PubMed
-
- Charlet-B. N, et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell. 2002;10:45–53. - PubMed
-
- Mankodi A, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell. 2002;10:35–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

