Chloride channel diseases resulting from impaired transepithelial transport or vesicular function
- PMID: 16075045
- PMCID: PMC1180548
- DOI: 10.1172/JCI25470
Chloride channel diseases resulting from impaired transepithelial transport or vesicular function
Abstract
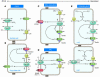
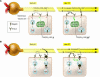
The transport of anions across cellular membranes is crucial for various functions, including the control of electrical excitability of muscle and nerve, transport of salt and water across epithelia, and the regulation of cell volume or the acidification and ionic homeostasis of intracellular organelles. Given this broad range of functions, it is perhaps not surprising that mutations in Cl- channels lead to a large spectrum of diseases. These diverse pathologies include the muscle disorder myotonia, cystic fibrosis, renal salt loss in Bartter syndrome, kidney stones, deafness, and the bone disease osteopetrosis. This review will focus on diseases related to transepithelial transport and on disorders involving vesicular Cl- channels.
Figures

References
-
- Hübner C, et al. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. - PubMed
-
- Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
-
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 2004;427:803–807. - PubMed
-
- Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. - PubMed
-
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nature. 1999;402:301–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

