A molecule's right to choose: how diabetogenic class II MHC products bind peptides
- PMID: 16075053
- PMCID: PMC1180559
- DOI: 10.1172/JCI26018
A molecule's right to choose: how diabetogenic class II MHC products bind peptides
Abstract
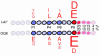
The distinction between peptides that bind to class II MHC products under laboratory conditions and those that do so physiologically is important for the prediction of antigens recognized by autoreactive T cells. In this issue of the JCI, Suri et al., using antigen-presenting cells, compared the peptides that bound to human HLA-DQ8 and those that bound to mouse I-A, both class II MHC products that predispose their carriers to type 1 diabetes. The rules of engagement for the peptide ligands of the DQ8 and I-A molecules involve similarities in their anchor residues, which mediate stable interaction with class II MHC products. The peptides identified derive from overlapping sets of self proteins.
Figures
Comment on
-
Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity.J Clin Invest. 2005 Aug;115(8):2268-76. doi: 10.1172/JCI25350. J Clin Invest. 2005. PMID: 16075062 Free PMC article.
References
-
- Kent SC, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–228. - PubMed
-
- Antoniou AN, Blackwood SL, Mazzeo D, Watts C. Control of antigen presentation by a single protease cleavage site. Immunity. 2000;12:391–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

