Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity
- PMID: 16075062
- PMCID: PMC1180544
- DOI: 10.1172/JCI25350
Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity
Abstract
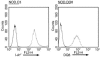
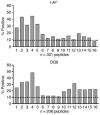
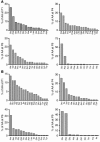
In this study, a large number of naturally processed peptides was isolated and identified from the human diabetes-susceptible class II MHC molecules HLA-DQ8 (DQA1*0301,DQB1*0302) and from murine I-A species, both of which are expressed in genetically identical APC lines. The peptides presented during the processing of autologous proteins were highly selective in showing sequence specificity, mainly consisting of 1 or more acidic residues at their C terminus. Testing for binding to the MHC molecules revealed that the position 9 (P9) acidic residues of the peptides contributed decisively to binding. For HLA-DQ8, the P1 residue, which was also an acidic amino acid, influenced binding positively. Both HLA-DQ8 and I-A(g7) selected for common peptides that bound in the same register. There was no evidence for selection of peptides having nonspecific or promiscuous binding. Thus, diabetogenic class II MHC molecules are highly selective in terms of the peptides presented by their APCs, and this is governed by the features of their P9 anchor pocket. These results are in striking contrast to those from studies examining synthetic peptide or phage display libraries, in which many peptides were shown to bind.
Figures
Comment in
-
A molecule's right to choose: how diabetogenic class II MHC products bind peptides.J Clin Invest. 2005 Aug;115(8):2077-9. doi: 10.1172/JCI26018. J Clin Invest. 2005. PMID: 16075053 Free PMC article.
References
-
- Castano L, Eisenbarth GS. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat [review] Annu. Rev. Immunol. 1990;8:647–679. - PubMed
-
- Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 1995;13:179–200. - PubMed
-
- McDevitt H, Singer S, Tisch R. The role of MHC class II genes in susceptibility and resistance to type I diabetes mellitus in the NOD mouse. Horm. Metab. Res. 1996;28:287–288. - PubMed
-
- Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains [review] Adv. Immunol. 1992;51:285–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

