Multiple roles of Rap1 in hematopoietic cells: complementary versus antagonistic functions
- PMID: 16076873
- PMCID: PMC1895320
- DOI: 10.1182/blood-2005-03-1062
Multiple roles of Rap1 in hematopoietic cells: complementary versus antagonistic functions
Abstract
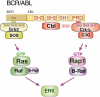
Small G proteins serve as critical control points in signal transduction, integrating a wide range of stimuli to dictate discrete cellular outcomes. The outcomes of small G-protein signaling can both potentiate and antagonize one another. Studies in hematopoietic cells have uncovered multiple functions for the small G protein, Rap1 (Ras-proximate-1). Because Rap1 can regulate cell proliferation, differentiation, and adhesion through distinct mechanisms, it serves as a paradigm for the need for tight cellular control of small G-protein function. Rap1 has received recent attention for its role in enhancing integrin-dependent signals. This action of Rap1 augments a variety of processes that characterize hematopoietic-cell function, including aggregation, migration, extravasation, and homing to target tissues. Rap1 may also regulate cellular differentiation and proliferation via pathways that are distinct from those mediating adhesion, and involve regulation of the mitogen-activated protein (MAP) kinase or ERK (extracellular signal-regulated kinase) cascade. These actions of Rap1 occur in selected cell types to enhance or diminish ERK signaling, depending on the expression pattern of the MAP kinase kinase kinases of the Raf family: Raf-1 and B-Raf. This review will examine the functions of Rap1 in hematopoietic cells, and focus on 3 cellular scenarios where the multiple actions of Rap1 function have been proposed. Recent studies implicating Rap1 in the maturation of megakaryocytes, the pathogenesis of chronic myelogenous leukemia (CML), and activation of peripheral T cells will receive particular attention.
Figures



References
-
- Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116: 435-440. - PubMed
-
- Katagiri K, Ohnishi N, Kabashima K, et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5: 1045-1051. - PubMed
-
- Ehrhardt A, Ehrhardt G, Guo X, Schrader J. Ras and relatives: job sharing and networking keep an old family together. Exp Hematol. 2002;30: 1089-1106. - PubMed
-
- Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat Immunol. 2004;5: 363-372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

