Long-lasting modifications of saccadic eye movements following adaptation induced in the double-step target paradigm
- PMID: 16077021
- PMCID: PMC1183262
- DOI: 10.1101/lm.96405
Long-lasting modifications of saccadic eye movements following adaptation induced in the double-step target paradigm
Abstract
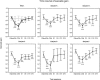
The adaptation of saccadic eye movements to environmental changes occurring throughout life is a good model of motor learning and motor memory. Numerous studies have analyzed the behavioral properties and neural substrate of oculomotor learning in short-term saccadic adaptation protocols, but to our knowledge, none have tested the persistence of the oculomotor memory. In the present study, the double-step target protocol was used in five human subjects to adaptively decrease the amplitude of reactive saccades triggered by a horizontally-stepping visual target. We tested the amplitude of visually guided saccades just before and at different times (up to 19 days) after the adaptation session. The results revealed that immediately after the adaptation session, saccade amplitude was significantly reduced by 22% on average. Although progressively recovering over days, this change in saccade gain was still statistically significant on days 1 and 5, with an average retention rate of 36% and 19%, respectively. On day 11, saccade amplitude no longer differed from the pre-adaptation value. Adaptation was more effective and more resistant to recovery for leftward saccades than for rightward ones. Lastly, modifications of saccade gain related to adaptation were accompanied by a decrease of both saccade duration and peak velocity. A control experiment indicated that all these findings were specifically related to the adaptation protocol, and further revealed that no change in the main sequence relationships could be specifically related to adaptation. We conclude that in humans, the modifications of saccade amplitude that quickly develop during a double-step target adaptation protocol can remain in memory for a much longer period of time, reflecting enduring plastic changes in the brain.
Figures






References
-
- Abel, L.A., Schmidt, D., Dell'Osso, L.F., and Daroff, R.B. 1978. Saccadic system plasticity in humans. Ann. Neurol. 4: 313-318. - PubMed
-
- Abrams, R.A., Dobkin, R.S., and Helfrich, M.K. 1992. Adaptive modification of saccadic eye movements. J. Exp. Psychol. Hum. Percept. Perform. 18: 922-933. - PubMed
-
- Alahyane, N. and Pélisson, D. 2004. Eye position specificity of saccadic adaptation. IOVS 45: 123-130. - PubMed
-
- ———. 2005. Retention of saccadic eye movements. Ann. NY Acad. Sci. 1039: 558-562. - PubMed
-
- Alahyane, N., Koene, A., and Pélisson, D. 2004. Transfer of adaptation from visually-guided saccades to averaging saccades elicited by double visual targets. Eur. J. Neurosci. 20: 827-836. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
