Deletion of the Mycobacterium tuberculosis pknH gene confers a higher bacillary load during the chronic phase of infection in BALB/c mice
- PMID: 16077122
- PMCID: PMC1196067
- DOI: 10.1128/JB.187.16.5751-5760.2005
Deletion of the Mycobacterium tuberculosis pknH gene confers a higher bacillary load during the chronic phase of infection in BALB/c mice
Abstract
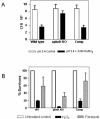
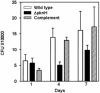
The role of the serine/threonine kinase PknH in the physiology and virulence of Mycobacterium tuberculosis was assessed by the construction of a pknH deletion mutant. Deletion of the pknH gene did not affect sensitivity to the antimycobacterial drug ethambutol, although it was previously thought to be involved in regulating expression of emb genes encoding arabinosyl transferases, the targets of ethambutol. Nevertheless, transcription analyses revealed that genes associated with mycobacterial cell wall component synthesis, such as emb and ini operons, are downstream substrates of the PknH signaling cascade. In vitro survival studies revealed that a mutant with a deletion of the pknH gene displayed increased resistance to acidified nitrite stress, suggesting that nitric oxide is one of the potential environmental triggers for PknH activation. The effect of pknH deletion on mycobacterial virulence was investigated in BALB/c mice. In this model, the DeltapknH mutant was found to survive and replicate to a higher bacillary load in mouse organs than its parental strain and the pknH-complemented strain. In contrast, another closely related kinase mutant, the DeltapknE mutant, obtained from the same parental strain, was not affected in its virulence phenotype. Infection of THP-1 cells or in vitro growth studies in 7H9 medium did not reveal a significant in vitro growth advantage phenotype for the DeltapknH mutant. In conclusion, we propose that the serine/threonine kinase PknH plays a role in regulating bacillary load in mouse organs to facilitate adaptation to the host environment, possibly by enabling a regulated chronic infection by M. tuberculosis.
Figures



References
-
- Alland, D., I. Kramnik, T. R. Weisbrod, L. Otsubo, R. Cerny, L. P. Miller, W. R. Jacobs, Jr., and B. R. Bloom. 1998. Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:13227-13232. - PMC - PubMed
-
- Av-Gay, Y., and M. Everett. 2000. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 8:238-244. - PubMed
-
- Bakal, C. J., and J. E. Davies. 2000. No longer an exclusive club: eukaryotic signalling domains in bacteria. Trends Cell Biol. 10:32-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

