Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans
- PMID: 16079065
- PMCID: PMC1280335
- DOI: 10.1289/ehp.8002
Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans
Abstract
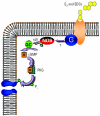
Glucagon, secreted from pancreatic alpha-cells integrated within the islets of Langerhans, is involved in the regulation of glucose metabolism by enhancing the synthesis and mobilization of glucose in the liver. In addition, it has other extrahepatic effects ranging from lipolysis in adipose tissue to the control of satiety in the central nervous system. In this article, we show that the endocrine disruptors bisphenol A (BPA) and diethylstilbestrol (DES), at a concentration of 10(-9) M, suppressed low-glucose-induced intracellular calcium ion ([Ca2+]i) oscillations in alpha-cells, the signal that triggers glucagon secretion. This action has a rapid onset, and it is reproduced by the impermeable molecule estradiol (E2) conjugated to horseradish peroxidase (E-HRP). Competition studies using E-HRP binding in immunocytochemically identified alpha-cells indicate that 17beta-E2, BPA, and DES share a common membrane-binding site whose pharmacologic profile differs from the classical ER. The effects triggered by BPA, DES, and E2 are blocked by the G alpha i- and G alpha o-protein inhibitor pertussis toxin, by the guanylate cyclase-specific inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, and by the nitric oxide synthase inhibitor N-nitro-L-arginine methyl ester. The effects are reproduced by 8-bromo-guanosine 3',5'-cyclic monophosphate and suppressed in the presence of the cGMP-dependent protein kinase inhibitor KT-5823. The action of E2, BPA, and DES in pancreatic alpha-cells may explain some of the effects elicited by endocrine disruptors in the metabolism of glucose and lipid.
Figures


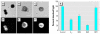
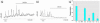
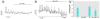
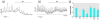



References
-
- Akesson B, Henningsson R, Salehi A, Lundquist I. Islet constitutive nitric oxide synthase and glucose regulation of insulin release in mice. J Endocrinol. 1999;163:39–48. - PubMed
-
- Berne RM, Levy MN. 1993. Physiology. St Louis:Mosby-Year Book, 867–871.
-
- Berts A, Ball A, Gylfe E, Hellman B. Suppression of Ca2+ oscillations in glucagon-producing alpha 2-cells by insulin/glucose and amino acids. Biochim Biophys Acta. 1996;1310:212–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
