Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal
- PMID: 16079072
- PMCID: PMC1280342
- DOI: 10.1289/ehp.7712
Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal
Abstract
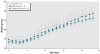
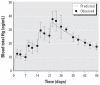
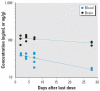
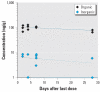
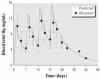
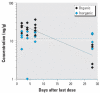
Thimerosal is a preservative that has been used in manufacturing vaccines since the 1930s. Reports have indicated that infants can receive ethylmercury (in the form of thimerosal) at or above the U.S. Environmental Protection Agency guidelines for methylmercury exposure, depending on the exact vaccinations, schedule, and size of the infant. In this study we compared the systemic disposition and brain distribution of total and inorganic mercury in infant monkeys after thimerosal exposure with those exposed to MeHg. Monkeys were exposed to MeHg (via oral gavage) or vaccines containing thimerosal (via intramuscular injection) at birth and 1, 2, and 3 weeks of age. Total blood Hg levels were determined 2, 4, and 7 days after each exposure. Total and inorganic brain Hg levels were assessed 2, 4, 7, or 28 days after the last exposure. The initial and terminal half-life of Hg in blood after thimerosal exposure was 2.1 and 8.6 days, respectively, which are significantly shorter than the elimination half-life of Hg after MeHg exposure at 21.5 days. Brain concentrations of total Hg were significantly lower by approximately 3-fold for the thimerosal-exposed monkeys when compared with the MeHg infants, whereas the average brain-to-blood concentration ratio was slightly higher for the thimerosal-exposed monkeys (3.5 +/- 0.5 vs. 2.5 +/- 0.3). A higher percentage of the total Hg in the brain was in the form of inorganic Hg for the thimerosal-exposed monkeys (34% vs. 7%). The results indicate that MeHg is not a suitable reference for risk assessment from exposure to thimerosal-derived Hg. Knowledge of the toxicokinetics and developmental toxicity of thimerosal is needed to afford a meaningful assessment of the developmental effects of thimerosal-containing vaccines.
Figures

References
-
- American Academy of Pediatrics and U.S. Public Health Service. Thimerosal in vaccines: a joint statement. MMWR Morb Mortal Wkly Rep. 1999;48:563–565. - PubMed
-
- Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in childhood vaccines. Pediatrics. 2001;107:1147–1154. - PubMed
-
- Berlin M, Crawford A, Grant DVM, Hellstrom J, Schultz A. Neurotoxicity of methylmercury in squirrel monkeys. Arch Environ Health. 1975;30:340–348. - PubMed
-
- Biroscak BJ, Fiore AE, Fasano N, Fineis P, Collins MP, Stoltman G. Impact of the thimerosal controversy on hepatitis B vaccine coverage of infants born to women of unknown hepatitis B surface antigen status in Michigan. Pediatrics. 2003;111:645–649. - PubMed
-
- Blaxill MF, Redwood L, Bernard S. Thimerosal and autism? A plausible hypothesis that should not be dismissed. Med Hypotheses. 2004;62:788–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
