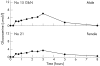
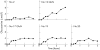Low levels of human serum glucosamine after ingestion of glucosamine sulphate relative to capability for peripheral effectiveness
- PMID: 16079170
- PMCID: PMC1798018
- DOI: 10.1136/ard.2005.036368
Low levels of human serum glucosamine after ingestion of glucosamine sulphate relative to capability for peripheral effectiveness
Abstract
Background: Oral glucosamine preparations are widely used as a treatment for osteoarthritis, purportedly functioning by a variety of mechanisms suggested by results of in vitro experiments, and generally using glucosamine concentrations well in excess of 100 micromol/l.
Objective: To use high performance liquid chromatography with a high sensitivity Metrohm-Peak instrument for pulsed amperometric measurement of human serum glucosamine; a detection limit of 0.5 micromol/l at 1:10 serum dilution allowed measurement of low levels of glucosamine in human serum, which previously has not been possible.
Methods: Eighteen subjects with osteoarthritis were given 1,500 mg of commercial glucosamine sulphate after an overnight fast, and serum was then obtained at baseline and every 15-30 minutes over 3 hours, and additionally, from two subjects at 5 and 8 hours. Urine samples were collected at baseline and 3 hours after ingestion from three subjects.
Results: Baseline glucosamine was below the detection limit of 0.5 mumol/l for all subjects, but after ingestion, glucosamine was detected in 17/18 subjects, beginning to rise at 30-45 minutes to a maximum at 90-180 minutes, with a range of 1.9-11.5 micromol/l (0.34-2 microg/ml).
Conclusion: This maximum concentration of 11.5 micromol/l has previously been shown to contribute less than 2% of the galactosamine incorporated into chondroitin sulphate in incubations of glucosamine with cultured human chondrocytes, and is a much lower concentration than the glucosamine concentrations claimed by other investigators to have various significant in vitro effects. This raises questions about current biological rationales for glucosamine use that were based on in vitro effects of glucosamine at much higher concentrations.
Conflict of interest statement
References
-
- Mili F, Helmick C G, Zack M M. Prevalence of arthritis: analysis of data from the US Behavioral Risk Factor Surveillance System, 1996–99. J Rheumatol 2002291981–1988. - PubMed
-
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati L. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3‐year, randomized, placebo‐controlled, double‐blind study. Arch Intern Med 20021622113–2123. - PubMed
-
- Reginster J Y, Deroisy R, Rovati L C, Lee R L, Lejeune E, Bruyere O.et al Long‐term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo‐controlled clinical trial. Lancet 2001357251–256. - PubMed
-
- McAlindon T E, LaValley M P, Gulin J P, Felson D T. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta‐analysis. JAMA 20002831469–1475. - PubMed
-
- Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin Y, Reginster J Y. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta‐analysis. Arch Intern Med 20031631514–1522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical