Role of TNF-{alpha} signaling in regeneration of cardiotoxin-injured muscle
- PMID: 16079187
- PMCID: PMC3099530
- DOI: 10.1152/ajpcell.00062.2005
Role of TNF-{alpha} signaling in regeneration of cardiotoxin-injured muscle
Abstract
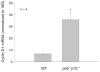
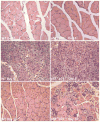
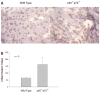
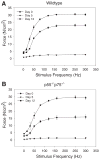
Recent data suggest a physiological role for the proinflammatory cytokine TNF-alpha in skeletal muscle regeneration. However, the underlying mechanism is not understood. In the present study, we analyzed TNF-alpha-activated signaling pathways involved in myogenesis in soleus muscle injured by cardiotoxin (CTX) in TNF-alpha receptor double-knockout mice (p55(-/-)p75(-/-)). We found that activation of p38MAPK, which is critical for myogenesis, was blocked in CTX-injured p55(-/-)p75(-/-) soleus on day 3 postinjury when myogenic differentiation was being initiated, while activation of ERK1/2 and JNK MAPK, as well as transcription factor NF-kappaB, was not reduced. Consequently, the phosphorylation of transcription factor myocyte enhancer factor-2C, which is catalyzed by p38 and crucial for the expression of muscle-specific genes, was blunted. Meanwhile, expression of p38-dependent differentiation marker myogenin and p21 were suppressed. In addition, expression of cyclin D1 was fivefold that in wild-type (WT) soleus. These results suggest that myogenic differentiation is blocked or delayed in the absence of TNF-alpha signaling. Histological studies revealed abnormalities in regenerating p55(-/-)p75(-/-) soleus. On day 5 postinjury, new myofiber formation was clearly observed in WT soleus but not in p55(-/-)p75(-/-) soleus. To the contrary, p55(-/-)p75(-/-) soleus displayed renewed inflammation and dystrophic calcification. On day 12 postinjury, the muscle architecture of WT soleus was largely restored. Yet, in p55(-/-)p75(-/-) soleus, multifocal areas of inflammation, myofiber death, and myofibers with smaller cross-sectional area were observed. Functional studies demonstrated an attenuated recovery of contractile force in injured p55(-/-)p75(-/-) soleus. These data suggest that TNF-alpha signaling plays a critical regulatory role in muscle regeneration.
Figures
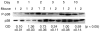



References
-
- Aronson D, Wojtaszewski JFP, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol Cell Physiol. 1998;275:C555–C561. - PubMed
-
- Cabane C, Englaro W, Yeow K, Ragno M, Dérijard B. Regulation of C2C12 myogenic terminal differentiation by MKK3/p38α pathway. Am J Physiol Cell Physiol. 2003;284:C658–C666. - PubMed
-
- Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol. 1995;54:121–128. - PubMed
-
- Cantini M, Massimino ML, Rapizzi E, Rossini K, Catani C, Dalla Libera L, Carraro U. Human satellite cell proliferation in vitro is regulated by autocrine secretion of IL-6 stimulated by a soluble factor(s) released by activated monocytes. Biochem Biophys Res Commun. 1995;216:49–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

