The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression
- PMID: 16079402
- PMCID: PMC6725237
- DOI: 10.1523/JNEUROSCI.1779-05.2005
The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression
Abstract
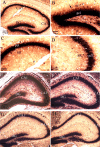
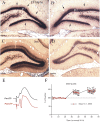
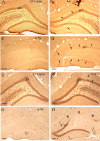
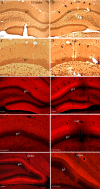
There is increasing evidence that long-lasting forms of activity-dependent synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), require local synthesis of proteins within dendrites. Identifying novel dendritic mRNAs and determining how their distribution and translation is regulated is a high priority. We demonstrate here that the mRNA for the elongation factor 1 alpha (EF1alpha) is present in vivo in the dendrites of neurons that exhibit LTP and LTD, and that its translation is locally regulated. The subcellular distribution of EF1alpha mRNA differs from any of the dendritic mRNAs that have been described previously. In the hippocampus, the mRNA is highly expressed in cell bodies and is also concentrated in the zone of termination of commissural/associational afferents in the inner molecular layer, suggesting that mRNA localization is in some way related to the distribution of different types of synapses. Nevertheless, the localization of EF1alpha mRNA is not altered by prolonged periods of synaptic activation that are sufficient to cause a dramatic redistribution of Arc mRNA. Local application of the metabotropic glutamate receptor agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) led to dramatic increases in immunostaining for EF1alpha protein in dendrites, and treatment of hippocampal slices with DHPG, which is known to induce LTD, led to increases in EF1alpha protein levels. Both responses were blocked by the protein synthesis inhibitor anisomycin. In contrast, stimulation of the perforant path using patterns of stimulation that induce LTP caused rapid increases of immunostaining for EF1alpha protein in the activated dendritic lamina, but these increases were not blocked by anisomycin or rapamycin. The findings suggest that local synthesis of EF1alpha protein may be important for the synaptic mechanisms that underlie protein synthesis-dependent LTD.
Figures





Comment in
-
A TOP at the Synapse.J Neurosci. 2005 Oct 26;25(43):9823-4. doi: 10.1523/JNEUROSCI.3612-05.2005. J Neurosci. 2005. PMID: 16251429 Free PMC article. No abstract available.
References
-
- Bailey CH, Kandel ER, Si K (2004) The persistence of long-term memory—a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron 44: 49-57. - PubMed
-
- Berkeley JL, Levey AI (2003) Cell-specific extracellular signal-regulated kinase activation by multiple G protein-coupled receptor families in hippocampus. Mol Pharmacol 63: 128-135. - PubMed
-
- Bliss TV, Collinridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31-39. - PubMed
-
- Camodeca N, Breakwell NA, Rowan MJ, Anwyl R (1999) Induction of LTD by activation of group I mGluR in the dentate gyrus in vitro. Neuropharmacology 38: 1579-1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
