Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity
- PMID: 16079404
- PMCID: PMC6725232
- DOI: 10.1523/JNEUROSCI.0909-05.2005
Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity
Abstract
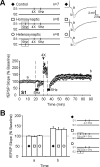
Long-term potentiation (LTP) is an enhancement of synaptic strength that may contribute to information storage in the mammalian brain. LTP expression can be regulated by previous synaptic activity, a process known as "metaplasticity." Cell-wide occurrence of metaplasticity may regulate synaptic strength. However, few reports have demonstrated metaplasticity at synapses that are silent during activity at converging synaptic inputs. We describe a novel form of cell-wide metaplasticity in hippocampal area CA1. Low-frequency stimulation (LFS) decreased the stability of long-lasting LTP ["late" LTP (L-LTP)] induced later at the same inputs (homosynaptic inhibition) and at other inputs converging on the same postsynaptic cells (heterosynaptic inhibition). Significantly, heterosynaptic inhibition of L-LTP also occurred across basal and apical dendrites ("heterodendritic" inhibition). Because transient early LTP (E-LTP) was not affected by previous LFS, we examined the effects of LFS on the consolidation of E-LTP to L-LTP. The duration of E-LTP induced at one set of inputs can be extended by capturing L-LTP-associated gene products generated by previous activity at other inputs to the same postsynaptic neurons. LFS applied homosynaptically or heterosynaptically before L-LTP induction did not impair synaptic capture by subsequent E-LTP stimulation, suggesting that LFS does not impair L-LTP-associated transcription. In contrast, LFS applied just before E-LTP (homosynaptically or heterosynaptically) prevented synaptic tagging, and capture of L-LTP expression. Thus, LFS inhibits synaptic tagging to impair expression of subsequent L-LTP. Such anterograde inhibition represents a novel way in which synaptic activity can regulate the expression of future long-lasting synaptic plasticity in a cell-wide manner.
Figures





References
-
- Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126-130. - PubMed
-
- Abraham WC, Robins A (2005) Memory retention—the synaptic stability versus plasticity dilemma. Trends Neurosci 28: 73-78. - PubMed
-
- Alger BE, Megela AL, Teyler TJ (1978) Transient heterosynaptic depression in the hippocampal slice. Brain Res Bull 3: 181-184. - PubMed
-
- Artola A, Singer W (1993) Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16: 480-487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
