Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy
- PMID: 16079410
- PMCID: PMC1820616
- DOI: 10.1523/JNEUROSCI.1879-05.2005
Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy
Abstract
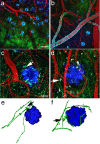
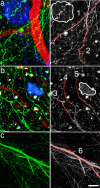
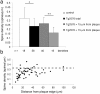
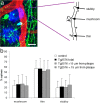
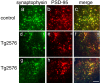
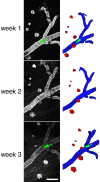
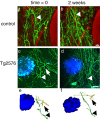
Accumulation of amyloid-beta (Abeta) into senile plaques in Alzheimer's disease (AD) is a hallmark neuropathological feature of the disorder, which likely contributes to alterations in neuronal structure and function. Recent work has revealed changes in neurite architecture associated with plaques and functional changes in cortical signaling in amyloid precursor protein (APP) expressing mouse models of AD. Here we developed a method using gene transfer techniques to introduce green fluorescent protein (GFP) into neurons, allowing the investigation of neuronal processes in the vicinity of plaques. Multiphoton imaging of GFP-labeled neurons in living Tg2576 APP mice revealed disrupted neurite trajectories and reductions in dendritic spine density compared with age-matched control mice. A profound deficit in spine density (approximately 50%) extends approximately 20 mum from plaque edges. Importantly, a robust decrement (approximately 25%) also occurs on dendrites not associated with plaques, suggesting widespread loss of postsynaptic apparatus. Plaques and dendrites remained stable over the course of weeks of imaging. Postmortem analysis of axonal immunostaining and colocalization of synaptophysin and postsynaptic density 95 protein staining around plaques indicate a parallel loss of presynaptic and postsynaptic partners. These results show considerable changes in dendrites and dendritic spines in APP transgenic mice, demonstrating a dramatic synaptotoxic effect of dense-cored plaques. Decreased spine density will likely contribute to altered neural system function and behavioral impairments observed in Tg2576 mice.
Figures

References
-
- Benzing WC, Mufson EJ, Armstrong DM (1993) Alzheimer's disease-like dystrophic neurites characteristically associated with senile plaques are not found within other neurodegenerative diseases unless amyloid beta-protein deposition is present. Brain Res 606: 10-18. - PubMed
-
- Berezovska O, Frosch M, McLean P, Knowles R, Koo E, Kang D, Shen J, Lu FM, Lux SE, Tonegawa S, Hyman BT (1999) The Alzheimer-related gene presenilin 1 facilitates notch 1 in primary mammalian neurons. Brain Res Mol Brain Res 69: 273-280. - PubMed
-
- Boncristiano S, Calhoun ME, Howard V, Bondolfi L, Kaeser SA, Wiederhold KH, Staufenbiel M, Jucker M (2005) Neocortical synaptic bouton number is maintained despite robust amyloid deposition in APP23 transgenic mice. Neurobiol Aging 26: 607-613. - PubMed
-
- Brendza RP, Simmons K, Bales KR, Paul SM, Goldberg MP, Holtzman DM (2003a) Use of YFP to study amyloid-beta associated neurite alterations in live brain slices. Neurobiol Aging 24: 1071-1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
