Archazolid and apicularen: novel specific V-ATPase inhibitors
- PMID: 16080788
- PMCID: PMC1190152
- DOI: 10.1186/1471-2091-6-13
Archazolid and apicularen: novel specific V-ATPase inhibitors
Abstract
Background: V-ATPases constitute a ubiquitous family of heteromultimeric, proton translocating proteins. According to their localization in a multitude of eukaryotic membranes, they energize many different transport processes. Since their malfunction is correlated with various diseases in humans, the elucidation of the properties of this enzyme for the development of selective inhibitors and drugs is one of the challenges in V-ATPase research.
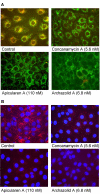
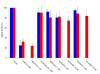
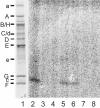
Results: Archazolid A and B, two recently discovered cytotoxic macrolactones produced by the myxobacterium Archangium gephyra, and apicularen A and B, two novel benzolactone enamides produced by different species of the myxobacterium Chondromyces, exerted a similar inhibitory efficacy on a wide range of mammalian cell lines as the well established plecomacrolidic type V-ATPase inhibitors concanamycin and bafilomycin. Like the plecomacrolides both new macrolides also prevented the lysosomal acidification in cells and inhibited the V-ATPase purified from the midgut of the tobacco hornworm, Manduca sexta, with IC50 values of 20-60 nM. However, they did not influence the activity of mitochondrial F-ATPase or that of the Na+/K+-ATPase. To define the binding sites of these new inhibitors we used a semi-synthetic radioactively labelled derivative of concanamycin which exclusively binds to the membrane Vo subunit c. Whereas archazolid A prevented, like the plecomacrolides concanamycin A, bafilomycin A1 and B1, labelling of subunit c by the radioactive I-concanolide A, the benzolactone enamide apicularen A did not compete with the plecomacrolide derivative.
Conclusion: The myxobacterial antibiotics archazolid and apicularen are highly efficient and specific novel inhibitors of V-ATPases. While archazolid at least partly shares a common binding site with the plecomacrolides bafilomycin and concanamycin, apicularen adheres to an independent binding site.
Figures


References
-
- Brown D, Smith PJ, Breton S. Role of V-ATPase-rich cells in acidification of the male reproductive tract. J Exp Biol. 1997;200:257–262. - PubMed
-
- Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers C, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nature Genetics. 1999;21:84–90. doi: 10.1038/5022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

