Comparative gene array analysis of TNF-alpha-induced MAPK and NF-kappaB signaling pathways between retinal ganglion cells and glial cells
- PMID: 16080915
- PMCID: PMC11824348
- DOI: 10.1016/j.exer.2005.01.022
Comparative gene array analysis of TNF-alpha-induced MAPK and NF-kappaB signaling pathways between retinal ganglion cells and glial cells
Abstract
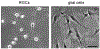
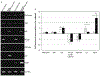
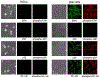
TNF-alpha has recently been identified to be a mediator of retinal ganglion cell (RGC) death, while glial cells are relatively protected against this death stimulus. To identify molecular mechanisms that control diverse responses of RGCs and glial cells to TNF-alpha, we studied differential gene expression between primary cultures of RGCs and glial cells exposed to TNF-alpha using cDNA array analysis of MAPK and NF-kappaB signaling pathways. Findings of this comparative analysis demonstrated differential regulation of various genes between RGCs and glial cells exposed to TNF-alpha. RT-PCR confirmed the differential expression of selected genes, and immunocytochemistry demonstrated gene products in cultured cells. Immunolabeling with phosphorylation site-specific antibodies also revealed differential post-translational modifications of selected proteins between cell types. Identification of signaling molecules differentially regulated in RGCs and glial cells can improve our understanding of the diverse cellular responses and provide targets for neuroprotective interventions in several neurodegenerative conditions.
Figures

Similar articles
-
Neuroprotection and neuroregeneration of retinal ganglion cells after intravitreal carbon monoxide release.PLoS One. 2017 Nov 27;12(11):e0188444. doi: 10.1371/journal.pone.0188444. eCollection 2017. PLoS One. 2017. PMID: 29176876 Free PMC article.
-
Tumor necrosis factor-alpha mediates activation of NF-κB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways.Eur J Neurosci. 2014 Oct;40(8):3171-8. doi: 10.1111/ejn.12710. Epub 2014 Aug 27. Eur J Neurosci. 2014. PMID: 25160799 Free PMC article.
-
Soluble tumor necrosis factor-alpha-induced hyperexcitability contributes to retinal ganglion cell apoptosis by enhancing Nav1.6 in experimental glaucoma.J Neuroinflammation. 2021 Aug 21;18(1):182. doi: 10.1186/s12974-021-02236-6. J Neuroinflammation. 2021. PMID: 34419081 Free PMC article.
-
Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells.Invest Ophthalmol Vis Sci. 2005 Aug;46(8):2983-91. doi: 10.1167/iovs.04-1177. Invest Ophthalmol Vis Sci. 2005. PMID: 16043875
-
TNF-alpha signaling in glaucomatous neurodegeneration.Prog Brain Res. 2008;173:409-21. doi: 10.1016/S0079-6123(08)01128-X. Prog Brain Res. 2008. PMID: 18929124 Free PMC article. Review.
Cited by
-
Polymorphism in the TNF-α(-863) locus associated with reduced risk of primary open angle glaucoma.Mol Vis. 2012;18:779-85. Epub 2012 Mar 31. Mol Vis. 2012. PMID: 22509108 Free PMC article.
-
An examination of the regulatory mechanism of Pxdn mutation-induced eye disorders using microarray analysis.Int J Mol Med. 2016 Jun;37(6):1449-56. doi: 10.3892/ijmm.2016.2572. Epub 2016 Apr 20. Int J Mol Med. 2016. PMID: 27121343 Free PMC article.
-
The role of aquaporin-4 in optic nerve head astrocytes in experimental glaucoma.PLoS One. 2021 Feb 2;16(2):e0244123. doi: 10.1371/journal.pone.0244123. eCollection 2021. PLoS One. 2021. PMID: 33529207 Free PMC article.
-
Oxidative stress and the regulation of complement activation in human glaucoma.Invest Ophthalmol Vis Sci. 2010 Oct;51(10):5071-82. doi: 10.1167/iovs.10-5289. Epub 2010 May 19. Invest Ophthalmol Vis Sci. 2010. PMID: 20484586 Free PMC article.
-
TGFβ signaling induces expression of Gadd45b in retinal ganglion cells.Invest Ophthalmol Vis Sci. 2013 Feb 5;54(2):1061-9. doi: 10.1167/iovs.12-10142. Invest Ophthalmol Vis Sci. 2013. PMID: 23329662 Free PMC article.
References
-
- Baeuerle PA, Baltimore D, 1996. NF-kappa B: ten years after. Cell 87, 13–20. - PubMed
-
- Baichwal VR, Baeuerle PA, 1997. Activate NF-kappa B or die?. Curr. Biol 7, R94–R96. - PubMed
-
- Bajramovic JJ, Bsibsi M, Geutskens SB, Hassankhan R, Verhulst KC, Stege GJ, de Groot CJ, van Noort JM, 2000. Differential expression of stress proteins in human adult astrocytes in response to cytokines. J. Neuroimmunol 106, 14–22. - PubMed
-
- Beg AA, Baltimore D, 1996. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 274, 782–784. - PubMed
-
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C, 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol 2, 645–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

