Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain
- PMID: 16080998
- PMCID: PMC3904737
- DOI: 10.1016/j.heares.2004.11.025
Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain
Abstract
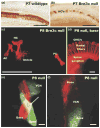
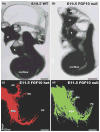
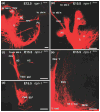
We review the in vivo evidence for afferent fiber guidance to the inner ear sensory epithelia and the central nuclei of termination. Specifically, we highlight our current molecular understanding for the role of hair cells and sensory epithelia in guiding afferents, how disruption of certain signals can alter fiber pathways, even in the presence of normal hair cells, and what role neurotrophins play in fiber guidance of sensory neurons to hair cells. The data suggest that the neurotrophin BDNF is the most important molecule known for inner ear afferent fiber guidance to hair cells in vivo. This suggestion is based on experiments on Ntf3 transgenic mice expressing BDNF under Ntf3 promoter that show deviations of fiber growth in the ear to areas that express BDNF but have no hair cells. However, fiber growth can occur in the absence of BDNF as demonstrated by double mutants for BDNF and Bax. We directly tested the significance of hair cells or sensory epithelia for fiber guidance in mutants that lose hair cells (Pou4f3) or do not form a posterior crista (Fgf10). While these data emphasize the role played by BDNF, normally released from hair cells, there is some limited capacity for directed growth even in the absence of hair cells, BDNF, or sensory epithelia. This directed growth may rely on semaphorins or other matrix proteins because targeted ablation of the sema3 docking site on the sema receptor Npn1 results in targeting errors of fibers even in the presence of hair cells and BDNF. Overall, our data support the notion that targeting of the afferent processes in the ear is molecularly distinct from targeting processes in the central nuclei. This conclusion is derived from data that show no recognizable central projection deviation, even if fibers are massively rerouted in the periphery, as in Ntf3(tgBDNF) mice in which vestibular fibers project to the cochlea.
Figures



Similar articles
-
Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance.Prog Brain Res. 2004;146:265-78. doi: 10.1016/S0079-6123(03)46017-2. Prog Brain Res. 2004. PMID: 14699969 Review.
-
Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention.Dev Dyn. 2005 Jun;233(2):570-83. doi: 10.1002/dvdy.20370. Dev Dyn. 2005. PMID: 15844198 Free PMC article.
-
Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation.BMC Neurosci. 2003 Jan 30;4:2. doi: 10.1186/1471-2202-4-2. BMC Neurosci. 2003. PMID: 12585968 Free PMC article.
-
A disorganized innervation of the inner ear persists in the absence of ErbB2.Brain Res. 2006 May 26;1091(1):186-99. doi: 10.1016/j.brainres.2006.02.090. Epub 2006 Apr 21. Brain Res. 2006. PMID: 16630588 Free PMC article. Review.
-
Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation.J Assoc Res Otolaryngol. 2000 Sep;1(2):129-43. doi: 10.1007/s101620010017. J Assoc Res Otolaryngol. 2000. PMID: 11545141 Free PMC article.
Cited by
-
Recent advances in hearing restoration.J R Soc Med. 2008 Mar;101(3):116-24. doi: 10.1258/jrsm.2007.070420. J R Soc Med. 2008. PMID: 18344468 Free PMC article. Review.
-
MEKK4 Signaling Regulates Sensory Cell Development and Function in the Mouse Inner Ear.J Neurosci. 2016 Jan 27;36(4):1347-61. doi: 10.1523/JNEUROSCI.1853-15.2016. J Neurosci. 2016. PMID: 26818521 Free PMC article.
-
Thyroid hormone is required for the pruning of afferent type II spiral ganglion neurons in the mouse cochlea.Neuroscience. 2016 Jan 15;312:165-78. doi: 10.1016/j.neuroscience.2015.11.020. Epub 2015 Nov 18. Neuroscience. 2016. PMID: 26592716 Free PMC article.
-
Otic mesenchyme cells regulate spiral ganglion axon fasciculation through a Pou3f4/EphA4 signaling pathway.Neuron. 2012 Jan 12;73(1):49-63. doi: 10.1016/j.neuron.2011.10.029. Neuron. 2012. PMID: 22243746 Free PMC article.
-
Wnts and Wnt inhibitors do not influence axon outgrowth from chicken statoacoustic ganglion neurons.Hear Res. 2011 Aug;278(1-2):86-95. doi: 10.1016/j.heares.2011.04.005. Epub 2011 Apr 22. Hear Res. 2011. PMID: 21530628 Free PMC article.
References
-
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Bianchi LM, Cohan CS. Developmental regulation of a neurite-promoting factor influencing statoacoustic neurons. Brain Res Dev Brain Res. 1991;64:167–174. - PubMed
-
- Bianchi LM, Cohan CS. Effects of the neurotrophins and CNTF on developing statoacoustic neurons: comparison with an otocyst-derived factor. Dev Biol. 1993;159:353–365. - PubMed
-
- Bianchi LM, Liu H. Comparison of ephrin-A ligand and EphA receptor distribution in the developing inner ear. Anat Rec. 1999;254:127–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

